Left Ventricular T-Cell Recruitment Contributes to the Pathogenesis of Heart Failure
- PMID: 26022677
- PMCID: PMC4512916
- DOI: 10.1161/CIRCHEARTFAILURE.115.002225
Left Ventricular T-Cell Recruitment Contributes to the Pathogenesis of Heart Failure
Abstract
Background: Despite the emerging association between heart failure (HF) and inflammation, the role of T cells, major players in chronic inflammation, has only recently begun to be explored. Whether T-cell recruitment to the left ventricle (LV) participates in the development of HF requires further investigation to identify novel mechanisms that may serve for the design of alternative therapeutic interventions.
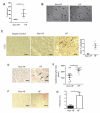
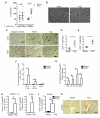
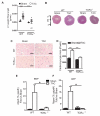
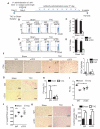
Methods and results: Real-time videomicroscopy of T cells from nonischemic HF patients or from mice with HF induced by transverse aortic constriction revealed enhanced adhesion to activated vascular endothelial cells under flow conditions in vitro compared with T cells from healthy subjects or sham mice. T cells in the mediastinal lymph nodes and the intramyocardial endothelium were both activated in response to transverse aortic constriction and the kinetics of LV T-cell infiltration was directly associated with the development of systolic dysfunction. In response to transverse aortic constriction, T cell-deficient mice (T-cell receptor, TCRα(-/-)) had preserved LV systolic and diastolic function, reduced LV fibrosis, hypertrophy and inflammation, and improved survival compared with wild-type mice. Furthermore, T-cell depletion in wild-type mice after transverse aortic constriction prevented HF.
Conclusions: T cells are major contributors to nonischemic HF. Their activation combined with the activation of the LV endothelium results in LV T-cell infiltration negatively contributing to HF progression through mechanisms involving cytokine release and induction of cardiac fibrosis and hypertrophy. Reduction of T-cell infiltration is thus identified as a novel translational target in HF.
Keywords: T lymphocytes; cell adhesion; heart failure; heart ventricles; inflammation; ventricular remodeling.
© 2015 American Heart Association, Inc.
Figures



References
-
- Cowie MR, Mosterd A, Wood DA, Deckers JW, Poole-Wilson PA, Sutton GC, Grobbee DE. The epidemiology of heart failure. Eur Heart J. 1997;18:208–25. - PubMed
-
- Lloyd-Jones D, Adams R, Carnethon M, De SG, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O’Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;11:480–6. - PubMed
-
- Ahmad T, Fiuzat M, Felker GM, O’Connor C. Novel biomarkers in chronic heart failure. Nat Rev Cardiol. 2012;9:347–59. - PubMed
-
- Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–18. - PubMed
-
- Aukrust P, Ueland T, Lien E, Bendtzen K, Muller F, Andreassen AK, Nordoy I, Aass H, Espevik T, Simonsen S, Froland SS, Gullestad L. Cytokine network in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1999;83:376–82. - PubMed
Publication types
MeSH terms
Grants and funding
- K08 HL094909/HL/NHLBI NIH HHS/United States
- R01-HL123658/HL/NHLBI NIH HHS/United States
- UL1 TR001064/TR/NCATS NIH HHS/United States
- K99-HL097406/HL/NHLBI NIH HHS/United States
- T32 HL069770/HL/NHLBI NIH HHS/United States
- T32 HL69770/HL/NHLBI NIH HHS/United States
- R00 HL094706/HL/NHLBI NIH HHS/United States
- R44 HL097406/HL/NHLBI NIH HHS/United States
- R01 HL123658/HL/NHLBI NIH HHS/United States
- KL2 TR001063/TR/NCATS NIH HHS/United States
- R43 HL097406/HL/NHLBI NIH HHS/United States
- 1KL2TR001063-01/TR/NCATS NIH HHS/United States
- UL1TR001064/TR/NCATS NIH HHS/United States
- K08HL094909/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

