Monocyte-endothelial cell interactions in the regulation of vascular sprouting and liver regeneration in mouse
- PMID: 26022689
- PMCID: PMC4575901
- DOI: 10.1016/j.jhep.2015.05.011
Monocyte-endothelial cell interactions in the regulation of vascular sprouting and liver regeneration in mouse
Abstract
Background & aims: Regeneration of the hepatic mass is crucial to liver repair. Proliferation of hepatic parenchyma is intimately dependent on angiogenesis and resident macrophage-derived cytokines. However the role of circulating monocyte interactions in vascular and hepatic regeneration is not well-defined. We investigated the role of these interactions in regeneration in the presence and absence of intact monocyte adhesion.
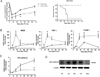
Methods: Partial hepatectomy was performed in wild-type mice and those lacking the monocyte adhesion molecule CD11b. Vascular architecture, angiogenesis and macrophage location were analyzed in the whole livers using simultaneous angiography and macrophage staining with fluorescent multiphoton microscopy. Monocyte adhesion molecule expression and sprouting-related pathways were evaluated.
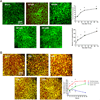
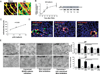
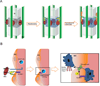
Results: Resident macrophages (Kupffer cells) did not migrate to interact with vessels whereas infiltrating monocytes were found adjacent to sprouting points. Infiltrated monocytes colocalized with Wnt5a, angiopoietin 1 and Notch-1 in contact points and commensurate with phosphorylation and disruption of VE-cadherin. Mice deficient in CD11b showed a severe reduction in angiogenesis, liver mass regeneration and survival following partial hepatectomy, and developed unstable and leaky vessels that eventually produced an aberrant hepatic vascular network and Kupffer cell distribution.
Conclusions: Direct vascular interactions of infiltrating monocytes are required for an ordered vascular growth and liver regeneration. These outcomes provide insight into hepatic repair and new strategies for hepatic regeneration.
Keywords: Angiogenesis; Hepatic repair; Immunology; Innate immune system.
Copyright © 2015 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures



References
-
- Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–S53. - PubMed
-
- Vollmar B, Menger MD. The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev. 2009;89:1269–1339. - PubMed
-
- Diehl AM. Neighborhood watch orchestrates liver regeneration. Nat Med. 2012;18:497–409. - PubMed
-
- Viebahn CS, Benseler V, Holz LE, Elsegood CL, Vo M, Bertolino P, et al. Invading macrophages play a major role in the liver progenitor cell response to chronic liver injury. J Hepatol. 2010;53:500–507. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

