Quantitative lipopolysaccharide analysis using HPLC/MS/MS and its combination with the limulus amebocyte lysate assay
- PMID: 26023073
- PMCID: PMC4479340
- DOI: 10.1194/jlr.D059725
Quantitative lipopolysaccharide analysis using HPLC/MS/MS and its combination with the limulus amebocyte lysate assay
Abstract
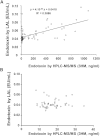
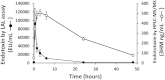
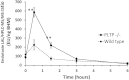
Quantitation of plasma lipopolysaccharides (LPSs) might be used to document Gram-negative bacterial infection. In the present work, LPS-derived 3-hydroxymyristate was extracted from plasma samples with an organic solvent, separated by reversed phase HPLC, and quantitated by MS/MS. This mass assay was combined with the limulus amebocyte lysate (LAL) bioassay to monitor neutralization of LPS activity in biological samples. The described HPLC/MS/MS method is a reliable, practical, accurate, and sensitive tool to quantitate LPS. The combination of the LAL and HPLC/MS/MS analyses provided new evidence for the intrinsic capacity of plasma lipoproteins and phospholipid transfer protein to neutralize the activity of LPS. In a subset of patients with systemic inflammatory response syndrome, with documented infection but with a negative plasma LAL test, significant amounts of LPS were measured by the HPLC/MS/MS method. Patients with the highest plasma LPS concentration were more severely ill. HPLC/MS/MS is a relevant method to quantitate endotoxin in a sample, to assess the efficacy of LPS neutralization, and to evaluate the proinflammatory potential of LPS in vivo.
Keywords: diagnostic tool; human; inflammation; lipid transfer protein; lipoprotein; liquid chromatography tandem mass spectrometry; mass spectrometry; mouse; sepsis; systemic inflammatory response syndrome.
Copyright © 2015 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Gibot S., Béné M. C., Noel R., Massin F., Guy J., Cravoisy A., Barraud D., De Carvalho Bittencourt M., Quenot J. P., Bollaert P. E., et al. 2012. Combination biomarkers to diagnose sepsis in the critically ill patient. Am. J. Respir. Crit. Care Med. 186: 65–71. - PubMed
-
- Beutler B., Rietschel E. T. 2003. Innate immune sensing and its roots: the story of endotoxin. Nat. Rev. Immunol. 3: 169–176. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

