Transcription factor PRDM8 is required for rod bipolar and type 2 OFF-cone bipolar cell survival and amacrine subtype identity
- PMID: 26023183
- PMCID: PMC4466745
- DOI: 10.1073/pnas.1505870112
Transcription factor PRDM8 is required for rod bipolar and type 2 OFF-cone bipolar cell survival and amacrine subtype identity
Abstract
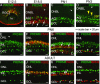
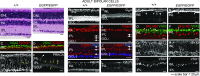
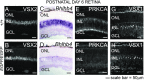
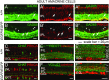
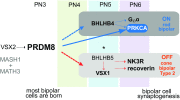
Retinal bipolar (BP) cells mediate the earliest steps in image processing in the visual system, but the genetic pathways that regulate their development and function are incompletely known. We identified PRDI-BF1 and RIZ homology domain containing 8 (PRDM8) as a highly conserved transcription factor that is abundantly expressed in mouse retina. During development and in maturity, PRDM8 is expressed strongly in BP cells and a fraction of amacrine and ganglion cells. To determine whether Prdm8 is essential to BP cell development or physiology, we targeted the gene in mice. Prdm8(EGFP/EGFP) mice showed nonprogressive b-wave deficits on electroretinograms, consistent with compromised BP cell function or circuitry resembling the incomplete form of human congenital stationary night blindness (CSNB). BP cell specification was normal in Prdm8(EGFP/EGFP) retina as determined by VSX2(+) cell numbers and retinal morphology at postnatal day 6. BP subtype differentiation was impaired, however, as indicated by absent or diminished expression of BP subtype-specific markers, including the putative PRDM8 regulatory target PKCα (Prkca) and its protein. By adulthood, rod bipolar (RB) and type 2 OFF-cone bipolar (CB) cells were nearly absent from Prdm8-null mice. Although no change was detected in total amacrine cell (AC) numbers, increased PRKCA(+) and cholinergic ACs and decreased GABAergic ACs were seen, suggesting an alteration in amacrine subtype identity. These findings establish that PRDM8 is required for RB and type 2 OFF-CB cell survival and amacrine subtype identity, and they present PRDM8 as a candidate gene for human CSNB.
Keywords: amacrine cell; bipolar cell; development; genetics; retina.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

