Direct observation of DNA overwinding by reverse gyrase
- PMID: 26023188
- PMCID: PMC4475935
- DOI: 10.1073/pnas.1422203112
Direct observation of DNA overwinding by reverse gyrase
Abstract
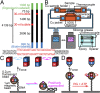
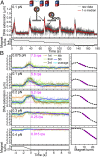
Reverse gyrase, found in hyperthermophiles, is the only enzyme known to overwind (introduce positive supercoils into) DNA. The ATP-dependent activity, detected at >70 °C, has so far been studied solely by gel electrophoresis; thus, the reaction dynamics remain obscure. Here, we image the overwinding reaction at 71 °C under a microscope, using DNA containing consecutive 30 mismatched base pairs that serve as a well-defined substrate site. A single reverse gyrase molecule processively winds the DNA for >100 turns. Bound enzyme shows moderate temperature dependence, retaining significant activity down to 50 °C. The unloaded reaction rate at 71 °C exceeds five turns per second, which is >10(2)-fold higher than hitherto indicated but lower than the measured ATPase rate of 20 s(-1), indicating loose coupling. The overwinding reaction sharply slows down as the torsional stress accumulates in DNA and ceases at stress of mere ∼ 5 pN ⋅ nm, where one more turn would cost only sixfold the thermal energy. The enzyme would thus keep DNA in a slightly overwound state to protect, but not overprotect, the genome of hyperthermophiles against thermal melting. Overwinding activity is also highly sensitive to DNA tension, with an effective interaction length exceeding the size of reverse gyrase, implying requirement for slack DNA. All results point to the mechanism where strand passage relying on thermal motions, as in topoisomerase IA, is actively but loosely biased toward overwinding.
Keywords: DNA overwinding; magnetic tweezers; reverse gyrase; topoisomerase; torsion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Kikuchi A, Asai K. Reverse gyrase—A topoisomerase which introduces positive superhelical turns into DNA. Nature. 1984;309(5970):677–681. - PubMed
-
- Suzuki T, et al. Sulfolobus tokodaii sp. nov. (f. Sulfolobus sp. strain 7), a new member of the genus Sulfolobus isolated from Beppu Hot Springs, Japan. Extremophiles. 2002;6(1):39–44. - PubMed
-
- Serre MC, Duguet M. Enzymes that cleave and religate DNA at high temperature: The same story with different actors. Prog Nucleic Acid Res Mol Biol. 2003;74:37–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

