Slow release anti-fungal skin formulations based on citric acid intercalated layered double hydroxides nanohybrids
- PMID: 26023319
- PMCID: PMC4446550
- DOI: 10.1186/s13065-015-0106-3
Slow release anti-fungal skin formulations based on citric acid intercalated layered double hydroxides nanohybrids
Abstract
Background: During the past few decades, the occurrence of superficial fungal infections has rapidly increased. As the fungal infections take longer time to get cured, concepts such as designing drugs with extended persistence and controlled release have gained attention. In this context, nanotechnology has been identified as the latest technological revolution which has opened up new pathways for designing new therapeutic materials. Out of the many available nano-structures layered double hydroxides have gained increased scientific attention in applications as slow and controlled release drug formulations. This study focuses on the encapsulation of citric acid which has anti-fungal properties into a Mg-Al- layered double hydroxide (LDH) in order to be used as slow release topical skin formulations.
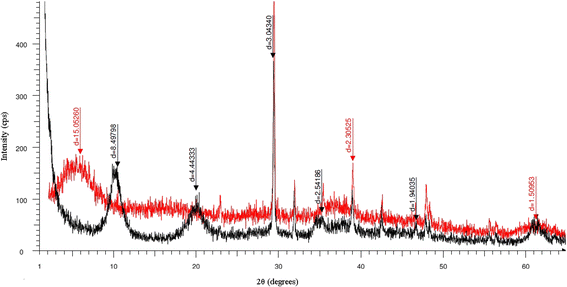
Results: Citrate ions were encapsulated into Mg-Al LDH using one step co-precipitation reaction. The successful intercalation of citrate ions into the layered structure has been proved referring to the expansion in the interlayer spacing as observed by the shift in the basal peak of the powder X-ray diffraction pattern. Fourier transform infra-red spectroscopy data suggests the change in the electron density around the carboxylate groups of the citrate ion thus providing evidences for formation of encapsulated hybrid composite. The resulting nanohybrid has been then, introduced into a general body cream formulation containing cocoa-butter. Both citrate LDH and the resulting body cream formulations demonstrated prolonged slow release characteristics up to 8 h in aqueous medium under different pH values (3, 4, and 5) compared to quick and fast release of pure citric acid. It was observed that the slow reelase was most efficient at low pH values. The encapsulation between the nano-layers and citrate ions are the key to the slow release characteristics. The body cream has been tested for the anti-fungal activity against three common Candida species (C. albicans, C. glabrata, C. tropicalis). The novel nanohybrid has shown an improved activity and slow release characteristics up to 48 h against the C. albicans and C. glabrata but not for C. tropicalis.
Conclusion: The study confirms that the citrate ion intercalated LDHs have the potential for use in future slow release antifungal drug formulation. Graphical AbstractSlow release nanohybrids based on citrate intercalated layered double hydroxides.
Keywords: Anti-fungal; Candida species; Citric acid; Layered double hydroxides; Nanohybrid; Slow release.
Figures






References
-
- Yang Y-L. Virulence factors of Candida species. J Microbiol Immunol Infect. 2003;36(4):223–228. - PubMed
-
- Del Hoyo C. Layered double hydroxides and human health: an overview. Appl Clay Sci. 2007;36:103–121. doi: 10.1016/j.clay.2006.06.010. - DOI
-
- Choy J, Choi S, Oh J, Park T. Clay minerals and layered double hydroxides for novel biological applications. Appl Clay Sci. 2007;36(1–3):122–132. doi: 10.1016/j.clay.2006.07.007. - DOI
-
- Reddy PD, Swarnalatha D. Recent advances in novel drug delivery systems. International Journal of PharmTech Research. 2010;2(3):2025–2027.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

