Rho GTPases as pathogen targets: Focus on curable sexually transmitted infections
- PMID: 26023809
- PMCID: PMC4601186
- DOI: 10.4161/21541248.2014.991233
Rho GTPases as pathogen targets: Focus on curable sexually transmitted infections
Abstract
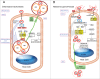
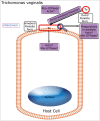
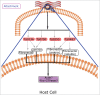
Pathogens have evolved highly specialized mechanisms to infect hosts. Several microorganisms modulate the eukaryotic cell surface to facilitate their engulfment. Once internalized, they hijack the molecular machinery of the infected cell for their own benefit. At different stages of phagocytosis, particularly during invasion, certain pathogens manipulate pathways governed by small GTPases. In this review, we focus on the role of Rho proteins on curable, sexually transmitted infections caused by Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis and Treponema pallidum. Despite the high, worldwide frequencies of these sexually-transmitted diseases, very little is known about the strategies developed by these microorganisms to usurp key eukaryotic proteins that control intracellular signaling and actin dynamics. Improved knowledge of these molecular mechanisms will contribute to the elucidation of how these clinically important pathogens manipulate intracellular processes and parasitize their hosts.
Keywords: Cdc42; Rac1; Rho; chlamydia trachomatis; neisseria gonorrhoeae; pathogen-host cell interaction; pathogens; small GTPases; treponema pallidum; trichomonas vaginalis.
Figures
References
-
- Rojas AM, Fuentes G, Rausell A, Valencia A. The ras protein superfamily: evolutionary tree and role of conserved amino acids. J Cell Biol 2012; 196:189-201; PMID:22270915; http://dx.doi.org/10.1083/jcb.201103008 - DOI - PMC - PubMed
-
- Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J 2000; 348 Pt 2:241-55; PMID:10816416; http://dx.doi.org/10.1042/0264-6021:3480241 - DOI - PMC - PubMed
-
- Thumkeo D, Watanabe S, Narumiya S. Physiological roles of rho and rho effectors in mammals. Eur J Cell Biol 2013; 92(10-11):303-15; PMID:24183240; http://dx.doi.org/10.1016/j.ejcb.2013.09.002 - DOI - PubMed
-
- Wennerberg K, Rossman KL, Der CJ. The ras superfamily at a glance. J Cell Sci 2005; 118:843-6; PMID:15731001; http://dx.doi.org/10.1242/jcs.01660 - DOI - PubMed
-
- Hall A. Rho GTPases and the actin cytoskeleton. Science 1998; 279:509-14; PMID:9438836; http://dx.doi.org/10.1126/science.279.5350.509 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
