A bioinspired peptide scaffold with high antibiotic activity and low in vivo toxicity
- PMID: 26024044
- PMCID: PMC4603705
- DOI: 10.1038/srep10558
A bioinspired peptide scaffold with high antibiotic activity and low in vivo toxicity
Abstract
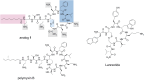
Bacterial resistance to almost all available antibiotics is an important public health issue. A major goal in antimicrobial drug discovery is the generation of new chemicals capable of killing pathogens with high selectivity, particularly multi-drug-resistant ones. Here we report the design, preparation and activity of new compounds based on a tunable, chemically accessible and upscalable lipopeptide scaffold amenable to suitable hit-to-lead development. Such compounds could become therapeutic candidates and future antibiotics available on the market. The compounds are cyclic, contain two D-amino acids for in vivo stability and their structures are reminiscent of other cyclic disulfide-containing peptides available on the market. The optimized compounds prove to be highly active against clinically relevant Gram-negative and Gram-positive bacteria. In vitro and in vivo tests show the low toxicity of the compounds. Their antimicrobial activity against resistant and multidrug-resistant bacteria is at the membrane level, although other targets may also be involved depending on the bacterial strain.
Figures

References
-
- Morel C. M. & Mossialos E. Stoking the antibiotic pipeline. British Med. J. 340, 1115–1118 (2010). - PubMed
-
- Cooper M. A. & Shlaes D. Fix the antibiotic pipeline. Nature 472, 32 (2011). - PubMed
-
- Butler M. S., Blaskovich M. A. & Cooper M. A. Antibiotics in the clinical pipeline in 2013. J. Antibiotics 66, 571–591 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

