Quantum dot labeling and tracking of cultured limbal epithelial cell transplants in vitro
- PMID: 26024089
- PMCID: PMC4506787
- DOI: 10.1167/iovs.14-15973
Quantum dot labeling and tracking of cultured limbal epithelial cell transplants in vitro
Abstract
Purpose: Cultured human limbal epithelial cells (HLECs) have shown promise in the treatment of limbal stem cell deficiency but little is known about their survival, behavior, and long-term fate after transplantation. The aim of this research was to evaluate, in vitro, quantum dot (Qdot) technology as a tool for tracking transplanted HLECs.
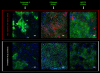
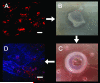
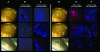
Methods: In vitro cultured HLECs were labeled with Qdot nanocrystals. Toxicity was assessed using live-dead assays. The effect on HLEC function was assessed using colony-forming efficiency assays and expression of CK3, P63alpha, and ABCG2. Sheets of cultured HLECs labeled with Qdot nanocrystals were transplanted onto decellularized human corneoscleral rims in an organ culture model and observed to investigate the behavior of transplanted cells.
Results: Quantum dot labeling had no detrimental effect on HLEC viability or function in vitro. Proliferation resulted in a gradual reduction in Qdot signal but sufficient signal was present to allow tracking of cells through multiple generations. Cells labeled with Qdots could be reliably detected and observed using confocal microscopy for at least 2 weeks after transplantation in our organ culture model. In addition, it was possible to label and observe epithelial cells in intact human corneas by using the Rostock corneal module adapted for use with the Heidelberg HRA.
Conclusions: This work demonstrates that Qdots combined with existing clinical equipment could be used to track HLEC for up to 2 weeks after transplantation; however, our model does not permit the assessment of cell labeling beyond 2 weeks. Further characterization in in vivo models are required.
Figures





Similar articles
-
Comparison of culture media for ex vivo cultivation of limbal epithelial progenitor cells.Mol Vis. 2013;19:69-77. Epub 2013 Jan 17. Mol Vis. 2013. PMID: 23378720 Free PMC article.
-
The use of human mesenchymal stem cell-derived feeder cells for the cultivation of transplantable epithelial sheets.Invest Ophthalmol Vis Sci. 2009 May;50(5):2109-15. doi: 10.1167/iovs.08-2262. Epub 2009 Jan 10. Invest Ophthalmol Vis Sci. 2009. PMID: 19136703
-
Ex vivo expanded SSEA-4+ human limbal stromal cells are multipotent and do not express other embryonic stem cell markers.Mol Vis. 2012;18:1289-300. Epub 2012 May 14. Mol Vis. 2012. PMID: 22665977 Free PMC article.
-
[Identification, purification and culture of limbal epithelial stem cells].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2009 Apr;26(2):452-6. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2009. PMID: 19499824 Review. Chinese.
-
Identification and characterization of limbal stem cells.Exp Eye Res. 2005 Sep;81(3):247-64. doi: 10.1016/j.exer.2005.02.016. Exp Eye Res. 2005. PMID: 16051216 Review.
Cited by
-
Near infra-red labelling and tracking of corneal endothelial cells in-vivo.Sci Rep. 2022 Apr 15;12(1):6338. doi: 10.1038/s41598-022-09677-w. Sci Rep. 2022. PMID: 35428788 Free PMC article.
-
Real-time assessment of corneal endothelial cell damage following graft preparation and donor insertion for DMEK.PLoS One. 2017 Oct 4;12(10):e0184824. doi: 10.1371/journal.pone.0184824. eCollection 2017. PLoS One. 2017. PMID: 28977017 Free PMC article.
-
Short- and Long-Term Results of Xenogeneic-Free Cultivated Autologous and Allogeneic Limbal Epithelial Stem Cell Transplantations.Cornea. 2019 Dec;38(12):1543-1549. doi: 10.1097/ICO.0000000000002153. Cornea. 2019. PMID: 31569145 Free PMC article.
-
Multimodal imaging quality control of epithelia regenerated with cultured human donor corneal limbal epithelial stem cells.Sci Rep. 2017 Jul 11;7(1):5154. doi: 10.1038/s41598-017-05486-8. Sci Rep. 2017. PMID: 28698576 Free PMC article.
-
Cellular structural and functional imaging of donor and pathological corneas with label-free dual-mode full-field optical coherence tomography.Biomed Opt Express. 2024 May 21;15(6):3869-3888. doi: 10.1364/BOE.525116. eCollection 2024 Jun 1. Biomed Opt Express. 2024. PMID: 38867788 Free PMC article.
References
-
- Baylis O, et al. 13 years of cultured limbal epithelial cell therapy: a review of the outcomes. Journal of cellular biochemistry. 2011;112(4):993–1002. - PubMed
-
- Shortt AJ, Tuft SJ, Daniels JT. Corneal stem cells in the eye clinic. British medical bulletin. 2011;100:209–25. - PubMed
-
- Rama P, et al. Limbal stem-cell therapy and long-term corneal regeneration. The New England journal of medicine. 2010;363(2):147–55. - PubMed
-
- Shortt AJ, et al. Transplantation of ex vivo cultured limbal epithelial stem cells: a review of techniques and clinical results. Surv.Ophthalmol. 2007;52(5):483–502. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

