Rat, mouse, and primate models of chronic glaucoma show sustained elevation of extracellular ATP and altered purinergic signaling in the posterior eye
- PMID: 26024091
- PMCID: PMC4439132
- DOI: 10.1167/iovs.14-15891
Rat, mouse, and primate models of chronic glaucoma show sustained elevation of extracellular ATP and altered purinergic signaling in the posterior eye
Abstract
Purpose: The cellular mechanisms linking elevated IOP with glaucomatous damage remain unresolved. Mechanical strains and short-term increases in IOP can trigger ATP release from retinal neurons and astrocytes, but the response to chronic IOP elevation is unknown. As excess extracellular ATP can increase inflammation and damage neurons, we asked if sustained IOP elevation was associated with a sustained increase in extracellular ATP in the posterior eye.
Methods: No ideal animal model of chronic glaucoma exists, so three different models were used. Tg-Myoc(Y437H) mice were examined at 40 weeks, while IOP was elevated in rats following injection of hypertonic saline into episcleral veins and in cynomolgus monkeys by laser photocoagulation of the trabecular meshwork. The ATP levels were measured using the luciferin-luciferase assay while levels of NTPDase1 were assessed using qPCR, immunoblots, and immunohistochemistry.
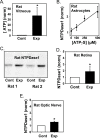
Results: The ATP levels were elevated in the vitreal humor of rats, mice, and primates after a sustained period of IOP elevation. The ecto-ATPase NTPDase1 was elevated in optic nerve head astrocytes exposed to extracellular ATP for an extended period. NTPDase1 was also elevated in the retinal tissue of rats, mice, and primates, and in the optic nerve of rats, with chronic elevation in IOP.
Conclusions: A sustained elevation in extracellular ATP, and upregulation of NTPDase1, occurs in the posterior eye of rat, mouse, and primate models of chronic glaucoma. This suggests the elevation in extracellular ATP may be sustained in chronic glaucoma, and implies a role for altered purinergic signaling in the disease.
Figures



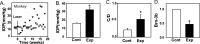

Comment in
-
Implications of sustained elevation in extracellular ATP in retina following chronic ocular hypertension.Invest Ophthalmol Vis Sci. 2015 May;56(5):3084. doi: 10.1167/iovs.15-17032. Invest Ophthalmol Vis Sci. 2015. PMID: 26024092 No abstract available.
References
-
- Osborne NN. Pathogenesis of ganglion “cell death” in glaucoma and neuroprotection: focus on ganglion cell axonal mitochondria. Prog Brain Res. 2008; 173: 339–352. - PubMed
-
- AGIS. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol. 2000; 130: 429–440. - PubMed
-
- Wax MB,, Tezel G. Immunoregulation of retinal ganglion cell fate in glaucoma. Exp Eye Res. 2009; 88: 825–830. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- EY10009/EY/NEI NIH HHS/United States
- P30 EY016665/EY/NEI NIH HHS/United States
- P30 EY010572/EY/NEI NIH HHS/United States
- EY016866/EY/NEI NIH HHS/United States
- MOP-93683/Canadian Institutes of Health Research/Canada
- EY016665/EY/NEI NIH HHS/United States
- K99 EY022077/EY/NEI NIH HHS/United States
- R01 EY010564/EY/NEI NIH HHS/United States
- EY015537/EY/NEI NIH HHS/United States
- EY10564/EY/NEI NIH HHS/United States
- R01 EY010145/EY/NEI NIH HHS/United States
- EY02698/EY/NEI NIH HHS/United States
- EY010145/EY/NEI NIH HHS/United States
- R01 EY015537/EY/NEI NIH HHS/United States
- EY001583/EY/NEI NIH HHS/United States
- Howard Hughes Medical Institute/United States
- P30 EY001583/EY/NEI NIH HHS/United States
- EY022077/EY/NEI NIH HHS/United States
- R01 EY016866/EY/NEI NIH HHS/United States
- R01 EY024259/EY/NEI NIH HHS/United States
- EY013434/EY/NEI NIH HHS/United States
- R00 EY022077/EY/NEI NIH HHS/United States
- R01 EY002698/EY/NEI NIH HHS/United States
- R01 EY010009/EY/NEI NIH HHS/United States
- R01 EY013434/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

