Involvement of corneal lymphangiogenesis in a mouse model of allergic eye disease
- PMID: 26024097
- PMCID: PMC4451613
- DOI: 10.1167/iovs.14-16186
Involvement of corneal lymphangiogenesis in a mouse model of allergic eye disease
Abstract
Purpose: The contribution of lymphangiogenesis (LA) to allergy has received considerable attention and therapeutic inhibition of this process via targeting VEGF has been considered. Likewise, certain inflammatory settings affecting the ocular mucosa can trigger pathogenic LA in the naturally avascular cornea. Chronic inflammation in allergic eye disease (AED) impacts the conjunctiva and cornea, leading to sight threatening conditions. However, whether corneal LA is involved is completely unknown. We addressed this using a validated mouse model of AED.
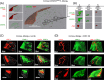
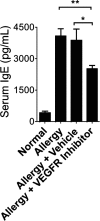
Methods: Allergic eye disease was induced by ovalbumin (OVA) immunization and chronic OVA exposure. Confocal microscopy of LYVE-1-stained cornea allowed evaluation of corneal LA, and qRT-PCR was used to evaluate expression of VEGF-C, -D, and -R3 in these mice. Administration of VEGF receptor (R) inhibitor was incorporated to inhibit corneal LA in AED. Immune responses were evaluated by in vitro OVA recall responses of T cells, and IgE levels in the serum.
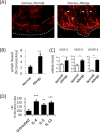
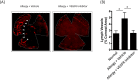
Results: Confocal microscopy of LYVE-1-stained cornea revealed the distinct presence of corneal LA in AED, and corroborated by increased corneal expression of VEGF-C, -D, and -R3. Importantly, prevention of corneal LA in AED via VEGFR inhibition was associated with decreased T helper two responses and IgE production. Furthermore, VEGFR inhibition led a significant reduction in clinical signs of AED.
Conclusions: Collectively, these data reveal that there is a distinct involvement of corneal LA in AED. Furthermore, VEGFR inhibition prevents corneal LA and consequent immune responses in AED.
Figures


Similar articles
-
Chronic inflammation, lymphangiogenesis, and effect of an anti-VEGFR therapy in a mouse model and in human patients with aspiration pneumonia.J Pathol. 2015 Mar;235(4):632-45. doi: 10.1002/path.4473. Epub 2015 Jan 7. J Pathol. 2015. PMID: 25348279
-
Evidence of corneal lymphangiogenesis in dry eye disease: a potential link to adaptive immunity?Arch Ophthalmol. 2010 Jul;128(7):819-24. doi: 10.1001/archophthalmol.2010.124. Arch Ophthalmol. 2010. PMID: 20625040 Free PMC article.
-
Lymphangiogenesis in aortic valve stenosis--novel regulatory roles for valvular myofibroblasts and mast cells.Atherosclerosis. 2012 Apr;221(2):366-74. doi: 10.1016/j.atherosclerosis.2011.12.034. Epub 2011 Dec 29. Atherosclerosis. 2012. PMID: 22281299
-
Molecular control of lymphatic metastasis.Ann N Y Acad Sci. 2008;1131:225-34. doi: 10.1196/annals.1413.020. Ann N Y Acad Sci. 2008. PMID: 18519975 Review.
-
Corneal lymphangiogenesis in herpetic stromal keratitis.Surv Ophthalmol. 2015 Jan-Feb;60(1):60-71. doi: 10.1016/j.survophthal.2014.06.001. Epub 2014 Jun 10. Surv Ophthalmol. 2015. PMID: 25444520 Free PMC article. Review.
Cited by
-
Ocular surface mast cells promote inflammatory lymphangiogenesis.Microvasc Res. 2022 May;141:104320. doi: 10.1016/j.mvr.2022.104320. Epub 2022 Jan 11. Microvasc Res. 2022. PMID: 35031275 Free PMC article.
-
Phase-specific functions of macrophages determine injury-mediated corneal hem- and lymphangiogenesis.Sci Rep. 2019 Jan 22;9(1):308. doi: 10.1038/s41598-018-36526-6. Sci Rep. 2019. PMID: 30670724 Free PMC article.
-
New targets of nascent lymphatic vessels in ocular diseases.Front Physiol. 2024 Mar 11;15:1374627. doi: 10.3389/fphys.2024.1374627. eCollection 2024. Front Physiol. 2024. PMID: 38529484 Free PMC article. Review.
-
The Plight of the Metabolite: Oxidative Stress and Tear Film Destabilisation Evident in Ocular Allergy Sufferers across Seasons in Victoria, Australia.Int J Mol Sci. 2024 Apr 4;25(7):4019. doi: 10.3390/ijms25074019. Int J Mol Sci. 2024. PMID: 38612830 Free PMC article.
-
Regulatory T Cells in Angiogenesis.J Immunol. 2020 Nov 15;205(10):2557-2565. doi: 10.4049/jimmunol.2000574. J Immunol. 2020. PMID: 33168598 Free PMC article. Review.
References
-
- Tammela T,, Alitalo K. Lymphangiogenesis: molecular mechanisms and future promise. Cell. 2010; 140: 460–476. - PubMed
-
- Alitalo K,, Tammela T,, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005; 438: 946–953. - PubMed
-
- Adams RH,, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007; 8: 464–478. - PubMed
-
- Kataru RP,, Jung K,, Jang C,, et al. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood. 2009; 113: 5650–5659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

