Validity of Automated Choroidal Segmentation in SS-OCT and SD-OCT
- PMID: 26024104
- PMCID: PMC4451615
- DOI: 10.1167/iovs.14-15669
Validity of Automated Choroidal Segmentation in SS-OCT and SD-OCT
Abstract
Purpose: To evaluate the validity of a novel fully automated three-dimensional (3D) method capable of segmenting the choroid from two different optical coherence tomography scanners: swept-source OCT (SS-OCT) and spectral-domain OCT (SD-OCT).
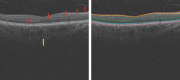
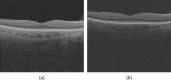
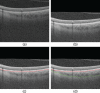
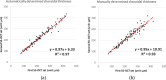
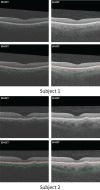
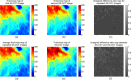
Methods: One hundred eight subjects were imaged using SS-OCT and SD-OCT. A 3D method was used to segment the choroid and quantify the choroidal thickness along each A-scan. The segmented choroidal posterior boundary was evaluated by comparing to manual segmentation. Differences were assessed to test the agreement between segmentation results of the same subject. Choroidal thickness was defined as the Euclidian distance between Bruch's membrane and the choroidal posterior boundary, and reproducibility was analyzed using automatically and manually determined choroidal thicknesses.
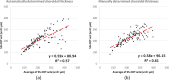
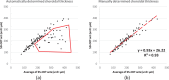
Results: For SS-OCT, the average choroidal thickness of the entire 6- by 6-mm2 macular region was 219.5 μm (95% confidence interval [CI], 204.9-234.2 μm), and for SD-OCT it was 209.5 μm (95% CI, 197.9-221.0 μm). The agreement between automated and manual segmentations was high: Average relative difference was less than 5 μm, and average absolute difference was less than 15 μm. Reproducibility of choroidal thickness between repeated SS-OCT scans was high (coefficient of variation [CV] of 3.3%, intraclass correlation coefficient [ICC] of 0.98), and differences between SS-OCT and SD-OCT results were small (CV of 11.0%, ICC of 0.73).
Conclusions: We have developed a fully automated 3D method for segmenting the choroid and quantifying choroidal thickness along each A-scan. The method yielded high validity. Our method can be used reliably to study local choroidal changes and may improve the diagnosis and management of patients with ocular diseases in which the choroid is affected.
Figures


References
-
- Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004; 122: 598–614. - PubMed
-
- Spaide RF. Age-related choroidal atrophy. Am J Ophthalmol. 2009; 147: 801–810. - PubMed
-
- Wang M,, Munch IC,, Hasler PW,, Prünte C,, Larsen M. Central serous chorioretinopathy. Acta Ophthalmol. 2008; 86: 126–145. - PubMed
-
- Ramrattan RS,, van der Schaft TL,, Mooy CM,, de Bruijn WC,, Mulder PG,, de Jong PT. Morphometric analysis of Bruch's membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci. 1994; 35: 2857–2864. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

