Cannabinoid-induced chemotaxis in bovine corneal epithelial cells
- PMID: 26024113
- PMCID: PMC4453295
- DOI: 10.1167/iovs.14-15675
Cannabinoid-induced chemotaxis in bovine corneal epithelial cells
Abstract
Purpose: Cannabinoid CB1 receptors are found in abundance in the vertebrate eye, with most tissue types expressing this receptor. However, the function of CB1 receptors in corneal epithelial cells (CECs) is poorly understood. Interestingly, the corneas of CB1 knockout mice heal more slowly after injury via a mechanism proposed to involve protein kinase B (Akt) activation, chemokinesis, and cell proliferation. The current study examined the role of cannabinoids in CEC migration in greater detail.
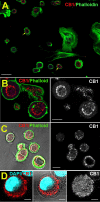
Methods: We determined the role of CB1 receptors in corneal healing. We examined the consequences of their activation on migration and proliferation in bovine CECs (bCECs). We additionally examined the mRNA profile of cannabinoid-related genes and CB1 protein expression as well as CB1 signaling in bovine CECs.
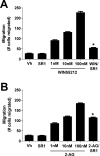
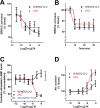
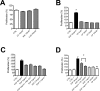
Results: We now report that activation of CB1 with physiologically relevant concentrations of the synthetic agonist WIN55212-2 (WIN) induces bCEC migration via chemotaxis, an effect fully blocked by the CB1 receptor antagonist SR141716. The endogenous agonist 2-arachidonoylglycerol (2-AG) also enhances migration. Separately, mRNA for most cannabinoid-related proteins are present in bovine corneal epithelium and cultured bCECs. Notably absent are CB2 receptors and the 2-AG synthesizing enzyme diglycerol lipase-α (DAGLα). The signaling profile of CB1 activation is complex, with inactivation of mitogen-activated protein kinase (MAPK). Lastly, CB1 activation does not induce bCEC proliferation, but may instead antagonize EGF-induced proliferation.
Conclusions: In summary, we find that CB1-based signaling machinery is present in bovine cornea and that activation of this system induces chemotaxis.
Figures


Similar articles
-
Cannabinoid receptor 1 suppresses transient receptor potential vanilloid 1-induced inflammatory responses to corneal injury.Cell Signal. 2013 Feb;25(2):501-11. doi: 10.1016/j.cellsig.2012.10.015. Epub 2012 Nov 8. Cell Signal. 2013. PMID: 23142606 Free PMC article.
-
Epidermal growth factor receptor transactivation by the cannabinoid receptor (CB1) and transient receptor potential vanilloid 1 (TRPV1) induces differential responses in corneal epithelial cells.Exp Eye Res. 2010 Sep;91(3):462-71. doi: 10.1016/j.exer.2010.06.022. Epub 2010 Jul 7. Exp Eye Res. 2010. PMID: 20619260 Free PMC article.
-
Cannabinoid CB2R receptors are upregulated with corneal injury and regulate the course of corneal wound healing.Exp Eye Res. 2019 May;182:74-84. doi: 10.1016/j.exer.2019.03.011. Epub 2019 Mar 21. Exp Eye Res. 2019. PMID: 30905716 Free PMC article.
-
Suppression of outward K⁺ currents by WIN55212-2 in rat retinal ganglion cells is independent of CB1/CB2 receptors.Neuroscience. 2013 Dec 3;253:183-93. doi: 10.1016/j.neuroscience.2013.08.056. Epub 2013 Sep 5. Neuroscience. 2013. PMID: 24013008
-
Cannabinoids in the treatment of cancer.Cancer Lett. 2009 Nov 18;285(1):6-12. doi: 10.1016/j.canlet.2009.04.005. Epub 2009 May 12. Cancer Lett. 2009. PMID: 19442435 Review.
Cited by
-
THC Regulates Tearing via Cannabinoid CB1 Receptors.Invest Ophthalmol Vis Sci. 2020 Aug 3;61(10):48. doi: 10.1167/iovs.61.10.48. Invest Ophthalmol Vis Sci. 2020. PMID: 32852544 Free PMC article.
-
Cannabinoid CB1 receptors regulate salivation.Sci Rep. 2022 Aug 19;12(1):14182. doi: 10.1038/s41598-022-17987-2. Sci Rep. 2022. PMID: 35986066 Free PMC article.
-
The role of the PI3K/AKT signalling pathway in the corneal epithelium: recent updates.Cell Death Dis. 2022 May 31;13(5):513. doi: 10.1038/s41419-022-04963-x. Cell Death Dis. 2022. PMID: 35641491 Free PMC article. Review.
-
Potential for endocannabinoid system modulation in ocular pain and inflammation: filling the gaps in current pharmacological options.Neuronal Signal. 2018 Nov 2;2(4):NS20170144. doi: 10.1042/NS20170144. eCollection 2018 Dec. Neuronal Signal. 2018. PMID: 32714590 Free PMC article. Review.
-
A Sex-Dependent Cannabinoid CB1 Receptor Role in Circadian Tearing of the Mouse.Invest Ophthalmol Vis Sci. 2024 Dec 2;65(14):10. doi: 10.1167/iovs.65.14.10. Invest Ophthalmol Vis Sci. 2024. PMID: 39630463 Free PMC article.
References
-
- Devane WA,, Hanus L,, Breuer A,, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992; 258: 1946–1949. - PubMed
-
- Stella N,, Schweitzer P,, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997; 388: 773–778. - PubMed
-
- Sugiura T,, Kondo S,, Sukagawa A, et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995; 215: 89–97. - PubMed
-
- Kano M,, Ohno-Shosaku T,, Hashimotodani Y,, Uchigashima M,, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009; 89: 309–380. - PubMed
-
- Howlett AC,, Breivogel CS,, Childers SR,, Deadwyler SA,, Hampson RE,, Porrino LJ. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology. 2004; 47(s uppl 1): 345–358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

