Hyperglycemia-suppressed expression of Serpine1 contributes to delayed epithelial wound healing in diabetic mouse corneas
- PMID: 26024123
- PMCID: PMC4453985
- DOI: 10.1167/iovs.15-16606
Hyperglycemia-suppressed expression of Serpine1 contributes to delayed epithelial wound healing in diabetic mouse corneas
Abstract
Purpose: Patients with diabetes mellitus (DM) are at an increased risk for developing corneal complications, including delayed wound healing. The purpose of this study was to characterize the expression and the function of Serpine1 and other components of urokinase plasminogen activator (uPA)-proteolytic system in delayed epithelial wound healing in diabetic mouse corneas.
Methods: Mice of the strain C57BL/6 were induced to develop diabetes by streptozotocin, and wound-healing assays were performed 10 weeks afterward. Gene expression and/or distribution were assessed by real-time PCR, Western blotting, and/or immunohistochemistry. The role of Serpine1 in mediating epithelial wound closure was determined by subconjunctival injections of neutralizing antibodies in either normal or recombinant protein in diabetic corneas. Enzyme assay for matrix metalloproteinase (MMP)-3 was also performed.
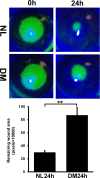
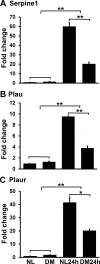
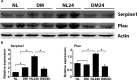
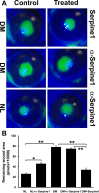
Results: The expressions of Serpine1 (PAI-1), Plau (uPA), and Plaur (uPA receptor) were upregulated in response to wounding, and these upregulations were significantly suppressed by hyperglycemia. In healing epithelia, Plau and Serpine1 were abundantly expressed at the leading edge of the healing epithelia of normal and, to a lesser extent, diabetic corneas. Inhibition of Serpine1 delayed epithelial wound closure in normal corneas, whereas recombinant Serpine1 accelerated it in diabetic corneas. The Plau and MMP-3 mRNA levels and MMP-3 enzymatic activities were correlated to Serpine1 levels and/or the rates of epithelial wound closure.
Conclusions: Serpine1 plays a role in mediating epithelial wound healing and its impaired expression may contribute to delayed wound healing in DM corneas. Hence, modulating uPA proteolytic pathway may represent a new approach for treating diabetic keratopathy.
Figures



References
-
- Clark EA,, Grabstein KH,, Gown AM,, et al. Activation of B lymphocyte maturation by a human follicular dendritic cell line, FDC-1. J Immunol. 1995; 155: 545–555. - PubMed
-
- Pflugfelder SC. Is autologous serum a tonic for the ailing corneal epithelium? Am J Ophthalmol. 2006; 142: 316–317. - PubMed
-
- Bikbova G,, Oshitari T,, Tawada A,, Yamamoto S. Corneal changes in diabetes mellitus. Curr Diabetes Rev. 2012; 8: 294–302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

