Recent advances in elucidating the genetic mechanisms of nephrogenesis using zebrafish
- PMID: 26024215
- PMCID: PMC4493457
- DOI: 10.3390/cells4020218
Recent advances in elucidating the genetic mechanisms of nephrogenesis using zebrafish
Abstract
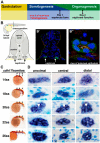
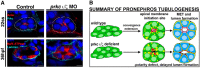
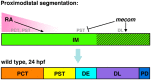
The kidney is comprised of working units known as nephrons, which are epithelial tubules that contain a series of specialized cell types organized into a precise pattern of functionally distinct segment domains. There is a limited understanding of the genetic mechanisms that establish these discrete nephron cell types during renal development. The zebrafish embryonic kidney serves as a simplified yet conserved vertebrate model to delineate how nephron segments are patterned from renal progenitors. Here, we provide a concise review of recent advances in this emerging field, and discuss how continued research using zebrafish genetics can be applied to gain insights about nephrogenesis.
Keywords: aPKC; hnf1ba/b; kidney development; mecom; nephron segmentation; renal progenitor; sim1a; tubulogenesis; vertebrate; zebrafish.
Figures
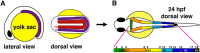
Similar articles
-
Homeogene emx1 is required for nephron distal segment development in zebrafish.Sci Rep. 2018 Dec 21;8(1):18038. doi: 10.1038/s41598-018-36061-4. Sci Rep. 2018. PMID: 30575756 Free PMC article.
-
Nephron proximal tubule patterning and corpuscles of Stannius formation are regulated by the sim1a transcription factor and retinoic acid in zebrafish.Dev Biol. 2015 Mar 1;399(1):100-116. doi: 10.1016/j.ydbio.2014.12.020. Epub 2014 Dec 25. Dev Biol. 2015. PMID: 25542995 Free PMC article.
-
Mechanisms of Nephrogenesis Revealed by Zebrafish Chemical Screen: Prostaglandin Signaling Modulates Nephron Progenitor Fate.Nephron. 2019;143(1):68-76. doi: 10.1159/000501037. Epub 2019 Jun 19. Nephron. 2019. PMID: 31216548 Free PMC article. Review.
-
Temporal and spatial expression of tight junction genes during zebrafish pronephros development.Gene Expr Patterns. 2014 Nov;16(2):104-13. doi: 10.1016/j.gep.2014.11.001. Epub 2014 Nov 7. Gene Expr Patterns. 2014. PMID: 25460834 Free PMC article.
-
Nephron Patterning: Lessons from Xenopus, Zebrafish, and Mouse Studies.Cells. 2015 Sep 11;4(3):483-99. doi: 10.3390/cells4030483. Cells. 2015. PMID: 26378582 Free PMC article. Review.
Cited by
-
Prostaglandin signaling regulates nephron segment patterning of renal progenitors during zebrafish kidney development.Elife. 2016 Dec 20;5:e17551. doi: 10.7554/eLife.17551. Elife. 2016. PMID: 27996936 Free PMC article.
-
Homeogene emx1 is required for nephron distal segment development in zebrafish.Sci Rep. 2018 Dec 21;8(1):18038. doi: 10.1038/s41598-018-36061-4. Sci Rep. 2018. PMID: 30575756 Free PMC article.
-
ppargc1a controls nephron segmentation during zebrafish embryonic kidney ontogeny.Elife. 2018 Nov 26;7:e40266. doi: 10.7554/eLife.40266. Elife. 2018. PMID: 30475208 Free PMC article.
-
Frataxin is essential for zebrafish embryogenesis and pronephros formation.Front Cell Dev Biol. 2024 Dec 11;12:1496244. doi: 10.3389/fcell.2024.1496244. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39723241 Free PMC article.
-
EF-hand domain containing 2 (Efhc2) is crucial for distal segmentation of pronephros in zebrafish.Cell Biosci. 2018 Oct 16;8:53. doi: 10.1186/s13578-018-0253-z. eCollection 2018. Cell Biosci. 2018. PMID: 30349665 Free PMC article.
References
-
- Hallgrimsson B., Benediktsson H., Vize P.D. Anatomy and histology of the human urinary system. In: Vize P.D., Woolf A.S., Bard J.B.L., editors. The Kidney, From Normal Development to Congenital Disease. Academic Press; London, UK: 2003. pp. 149–164.
-
- Reilly R.F., Bulger R.E., Kriz W. Structural-functional relationships in the kidney. In: Schrier R.W., editor. Diseases of the Kidney and Urinary Tract. 8th ed. Volume 1. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2007. pp. 2–53.
-
- Saxen L. Organogenesis of the Kidney. Cambridge University Press; Cambridge, UK: 1987.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

