Ex vivo host and parasite response to antileishmanial drugs and immunomodulators
- PMID: 26024228
- PMCID: PMC4449175
- DOI: 10.1371/journal.pntd.0003820
Ex vivo host and parasite response to antileishmanial drugs and immunomodulators
Abstract
Background: Therapeutic response in infectious disease involves host as well as microbial determinants. Because the immune and inflammatory response to Leishmania (Viannia) species defines the outcome of infection and efficacy of treatment, immunomodulation is considered a promising therapeutic strategy. However, since Leishmania infection and antileishmanial drugs can themselves modulate drug transport, metabolism and/or immune responses, immunotherapeutic approaches require integrated assessment of host and parasite responses.
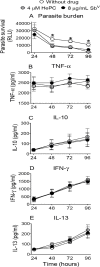
Methodology: To achieve an integrated assessment of current and innovative therapeutic strategies, we determined host and parasite responses to miltefosine and meglumine antimoniate alone and in combination with pentoxifylline or CpG 2006 in peripheral blood mononuclear cells (PBMCs) of cutaneous leishmaniasis patients. Parasite survival and secretion of TNF-α, IFN-γ, IL-10 and IL-13 were evaluated concomitantly in PBMCs infected with Luc-L. (V.) panamensis exposed to meglumine antimoniate (4, 8, 16, 32 and 64 μg SbV/mL) or miltefosine (2, 4, 8, 16 and 32 μM HePC). Concentrations of 4 μM of miltefosine and 8 μg SbV/mL were selected for evaluation in combination with immunomodulators based on the high but partial reduction of parasite burden by these antileishmanial concentrations without affecting cytokine secretion of infected PBMCs. Intracellular parasite survival was determined by luminometry and cytokine secretion measured by ELISA and multiplex assays.
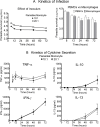
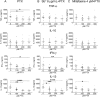
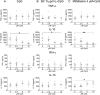
Principal findings: Anti- and pro-inflammatory cytokines characteristic of L. (V.) panamensis infection were evaluable concomitantly with viability of Leishmania within monocyte-derived macrophages present in PBMC cultures. Both antileishmanial drugs reduced the parasite load of macrophages; miltefosine also suppressed IL-10 and IL-13 secretion in a dose dependent manner. Pentoxifylline did not affect parasite survival or alter antileishmanial effects of miltefosine or meglumine antimoniate. However, pentoxifylline diminished secretion of TNF-α, IFN-γ and IL-13, cytokines associated with the outcome of infection by species of the Viannia subgenus. Exposure to CpG diminished the leishmanicidal effect of meglumine antimoniate, but not miltefosine, and significantly reduced secretion of IL-10, alone and in combination with either antileishmanial drug. IL-13 increased in response to CpG plus miltefosine.
Conclusions and significance: Human PBMCs allow integrated ex vivo assessment of antileishmanial treatments, providing information on host and parasite determinants of therapeutic response that may be used to tailor therapeutic strategies to optimize clinical resolution.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Novel approach to in vitro drug susceptibility assessment of clinical strains of Leishmania spp.J Clin Microbiol. 2012 Jul;50(7):2207-11. doi: 10.1128/JCM.00216-12. Epub 2012 Apr 18. J Clin Microbiol. 2012. PMID: 22518860 Free PMC article.
-
Natural resistance to meglumine antimoniate is associated with treatment failure in cutaneous leishmaniasis caused by Leishmania (Viannia) panamensis.PLoS Negl Trop Dis. 2024 May 6;18(5):e0012156. doi: 10.1371/journal.pntd.0012156. eCollection 2024 May. PLoS Negl Trop Dis. 2024. PMID: 38709850 Free PMC article.
-
Miltefosine and antimonial drug susceptibility of Leishmania Viannia species and populations in regions of high transmission in Colombia.PLoS Negl Trop Dis. 2014 May 22;8(5):e2871. doi: 10.1371/journal.pntd.0002871. eCollection 2014 May. PLoS Negl Trop Dis. 2014. PMID: 24853871 Free PMC article.
-
Systematic Review of Host-Mediated Activity of Miltefosine in Leishmaniasis through Immunomodulation.Antimicrob Agents Chemother. 2019 Jun 24;63(7):e02507-18. doi: 10.1128/AAC.02507-18. Print 2019 Jul. Antimicrob Agents Chemother. 2019. PMID: 31036692 Free PMC article.
-
Anticancer compounds as leishmanicidal drugs: challenges in chemotherapy and future perspectives.Curr Med Chem. 2008;15(5):433-9. doi: 10.2174/092986708783503221. Curr Med Chem. 2008. PMID: 18288998 Review.
Cited by
-
Route map for the discovery and pre-clinical development of new drugs and treatments for cutaneous leishmaniasis.Int J Parasitol Drugs Drug Resist. 2019 Dec;11:106-117. doi: 10.1016/j.ijpddr.2019.06.003. Epub 2019 Jun 20. Int J Parasitol Drugs Drug Resist. 2019. PMID: 31320296 Free PMC article. Review.
-
In vitro and in vivo immunomodulatory properties of octyl-β-D-galactofuranoside during Leishmania donovani infection.Parasit Vectors. 2019 Dec 23;12(1):600. doi: 10.1186/s13071-019-3858-0. Parasit Vectors. 2019. PMID: 31870416 Free PMC article.
-
Potency and Preclinical Evidence of Synergy of Oral Azole Drugs and Miltefosine in an Ex Vivo Model of Leishmania (Viannia) panamensis Infection.Antimicrob Agents Chemother. 2022 Jan 18;66(1):e0142521. doi: 10.1128/AAC.01425-21. Epub 2021 Oct 25. Antimicrob Agents Chemother. 2022. PMID: 34694879 Free PMC article.
-
The Src kinases Hck, Fgr and Lyn activate Arg to facilitate IgG-mediated phagocytosis and Leishmania infection.J Cell Sci. 2016 Aug 15;129(16):3130-43. doi: 10.1242/jcs.185595. Epub 2016 Jun 29. J Cell Sci. 2016. PMID: 27358479 Free PMC article.
-
Host, parasite and drug determinants of clinical outcomes following treatment of visceral leishmaniasis: a protocol for individual participant data meta-analysis.BMJ Open. 2023 Oct 28;13(10):e074679. doi: 10.1136/bmjopen-2023-074679. BMJ Open. 2023. PMID: 37898487 Free PMC article.
References
-
- Elting LS, Rubenstein EB, Rolston KVI, Bodey GP. Outcomes of Bacteremia in Patients with Cancer and Neutropenia: Observations from Two Decades of Epidemiological and Clinical Trials. Clinical Infectious Diseases1997. p. 247–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

