Acyclovir Has Low but Detectable Influence on HLA-B*57:01 Specificity without Inducing Hypersensitivity
- PMID: 26024233
- PMCID: PMC4449000
- DOI: 10.1371/journal.pone.0124878
Acyclovir Has Low but Detectable Influence on HLA-B*57:01 Specificity without Inducing Hypersensitivity
Abstract
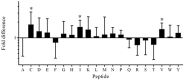
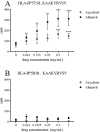
Immune mediated adverse drug reactions (IM-ADRs) remain a significant source of patient morbidity that have more recently been shown to be associated with specific class I and/or II human leukocyte antigen (HLA) alleles. Abacavir-induced hypersensitivity syndrome is a CD8+ T cell dependent IM-ADR that is exclusively mediated by HLA-B*57:01. We and others have previously shown that abacavir can occupy the floor of the peptide binding groove of HLA-B*57:01 molecules, increasing the affinity of certain self peptides resulting in an altered peptide-binding repertoire. Here, we have identified another drug, acyclovir, which appears to act in a similar fashion. As with abacavir, acyclovir showed a dose dependent increase in affinity for peptides with valine and isoleucine at their C-terminus. In agreement with the binding studies, HLA-B*57:01 peptide-elution studies performed in the presence of acyclovir revealed an increased number of endogenously bound peptides with a C-terminal isoleucine. Accordingly, we have hypothesized that acyclovir acts by the same mechanism as abacavir, although our data also suggest the overall effect is much smaller: the largest changes of peptide affinity for acyclovir were 2-5 fold, whereas for abacavir this effect was as much as 1000-fold. Unlike abacavir, acyclovir is not known to cause IM-ADRs. We conclude that the modest effect of acyclovir on HLA binding affinity in contrast to the large effect of abacavir is insufficient to trigger a hypersensitivity syndrome. We further support this by functional in vitro studies where acyclovir, unlike abacavir, was unable to produce an increase in IFN-γ upon expansion of HLA-B*57:01+ PBMCs from healthy donors. Using abacavir and acyclovir as examples we therefore propose an in vitro pre-clinical screening strategy, whereby thresholds can be applied to MHC-peptide binding assays to determine the likelihood that a drug could cause a clinically relevant IM-ADR.
Conflict of interest statement
Figures



Similar articles
-
Abacavir induces loading of novel self-peptides into HLA-B*57: 01: an autoimmune model for HLA-associated drug hypersensitivity.AIDS. 2012 Jul 17;26(11):F21-9. doi: 10.1097/QAD.0b013e328355fe8f. AIDS. 2012. PMID: 22617051 Free PMC article.
-
The Role of Conformational Dynamics in Abacavir-Induced Hypersensitivity Syndrome.Sci Rep. 2019 Jul 19;9(1):10523. doi: 10.1038/s41598-019-47001-1. Sci Rep. 2019. PMID: 31324847 Free PMC article.
-
Kinetics of Abacavir-Induced Remodelling of the Major Histocompatibility Complex Class I Peptide Repertoire.Front Immunol. 2021 May 19;12:672737. doi: 10.3389/fimmu.2021.672737. eCollection 2021. Front Immunol. 2021. PMID: 34093574 Free PMC article.
-
HLAs: Key regulators of T-cell-mediated drug hypersensitivity.HLA. 2018 Jan;91(1):3-16. doi: 10.1111/tan.13183. HLA. 2018. PMID: 29171940 Free PMC article. Review.
-
Studies on abacavir-induced hypersensitivity reaction: a successful example of translation of pharmacogenetics to personalized medicine.Sci China Life Sci. 2013 Feb;56(2):119-24. doi: 10.1007/s11427-013-4438-8. Epub 2013 Feb 8. Sci China Life Sci. 2013. PMID: 23393027 Review.
Cited by
-
Adverse drug reactions triggered by the common HLA-B*57:01 variant: a molecular docking study.J Cheminform. 2017 Mar 4;9:13. doi: 10.1186/s13321-017-0202-6. eCollection 2017. J Cheminform. 2017. PMID: 28303164 Free PMC article.
-
Adverse drug reactions triggered by the common HLA-B*57:01 variant: virtual screening of DrugBank using 3D molecular docking.J Cheminform. 2018 Jan 30;10(1):3. doi: 10.1186/s13321-018-0257-z. J Cheminform. 2018. PMID: 29383457 Free PMC article.
-
Computational Prediction of Potential Inhibitors of the Main Protease of SARS-CoV-2.Front Chem. 2020 Dec 23;8:590263. doi: 10.3389/fchem.2020.590263. eCollection 2020. Front Chem. 2020. PMID: 33425850 Free PMC article.
-
Repurposing Cardio-Metabolic Drugs to Fight Covid19.High Blood Press Cardiovasc Prev. 2021 Sep;28(5):419-423. doi: 10.1007/s40292-021-00475-5. Epub 2021 Sep 15. High Blood Press Cardiovasc Prev. 2021. PMID: 34524680 Free PMC article. No abstract available.
-
T-cell-mediated drug hypersensitivity: immune mechanisms and their clinical relevance.Asia Pac Allergy. 2016 Apr;6(2):77-89. doi: 10.5415/apallergy.2016.6.2.77. Epub 2016 Apr 28. Asia Pac Allergy. 2016. PMID: 27141480 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

