Hepatitis C virus and autophagy
- PMID: 26024249
- PMCID: PMC4778564
- DOI: 10.1515/hsz-2015-0172
Hepatitis C virus and autophagy
Abstract
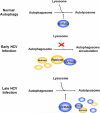
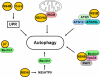
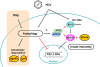
Autophagy is a catabolic process by which cells remove protein aggregates and damaged organelles for recycling. It can also be used by cells to remove intracellular microbial pathogens, including viruses, in a process known as xenophagy. However, many viruses have developed mechanisms to subvert this intracellular antiviral response and even use this pathway to support their own replications. Hepatitis C virus (HCV) is one such virus and is an important human pathogen that can cause severe liver diseases. Recent studies indicated that HCV could activate the autophagic pathway to support its replication. This review summarizes the current knowledge on the interplay between HCV and autophagy and how this interplay affects HCV replication and host innate immune responses.
Figures
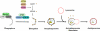
Similar articles
-
Hepatitis C Virus-Induced Autophagy and Host Innate Immune Response.Viruses. 2017 Aug 12;9(8):224. doi: 10.3390/v9080224. Viruses. 2017. PMID: 28805674 Free PMC article. Review.
-
Autophagy and Mammalian Viruses: Roles in Immune Response, Viral Replication, and Beyond.Adv Virus Res. 2016;95:149-95. doi: 10.1016/bs.aivir.2016.02.002. Epub 2016 Mar 10. Adv Virus Res. 2016. PMID: 27112282 Review.
-
Suppression of Host Innate Immune Response by Hepatitis C Virus via Induction of Autophagic Degradation of TRAF6.J Virol. 2016 Nov 14;90(23):10928-10935. doi: 10.1128/JVI.01365-16. Print 2016 Dec 1. J Virol. 2016. PMID: 27681126 Free PMC article.
-
Hepatitis C virus enhances Rubicon expression, leading to autophagy inhibition and intracellular innate immune activation.Sci Rep. 2020 Sep 17;10(1):15290. doi: 10.1038/s41598-020-72294-y. Sci Rep. 2020. PMID: 32943718 Free PMC article.
-
HCV-induced autophagy and innate immunity.Front Immunol. 2024 Feb 2;15:1305157. doi: 10.3389/fimmu.2024.1305157. eCollection 2024. Front Immunol. 2024. PMID: 38370419 Free PMC article. Review.
Cited by
-
Endoplasmic Reticulum: The Favorite Intracellular Niche for Viral Replication and Assembly.Viruses. 2016 Jun 7;8(6):160. doi: 10.3390/v8060160. Viruses. 2016. PMID: 27338443 Free PMC article. Review.
-
An Update on the Study of the Molecular Mechanisms Involved in Autophagy during Bacterial Pathogenesis.Biomedicines. 2024 Aug 5;12(8):1757. doi: 10.3390/biomedicines12081757. Biomedicines. 2024. PMID: 39200221 Free PMC article. Review.
-
Hepatitis C Virus Induces the Localization of Lipid Rafts to Autophagosomes for Its RNA Replication.J Virol. 2017 Sep 27;91(20):e00541-17. doi: 10.1128/JVI.00541-17. Print 2017 Oct 15. J Virol. 2017. PMID: 28747506 Free PMC article.
-
HCV and Oxidative Stress: Implications for HCV Life Cycle and HCV-Associated Pathogenesis.Oxid Med Cell Longev. 2016;2016:9012580. doi: 10.1155/2016/9012580. Epub 2016 Feb 3. Oxid Med Cell Longev. 2016. PMID: 26955431 Free PMC article. Review.
-
HCV-induced autophagosomes are generated via homotypic fusion of phagophores that mediate HCV RNA replication.PLoS Pathog. 2017 Sep 19;13(9):e1006609. doi: 10.1371/journal.ppat.1006609. eCollection 2017 Sep. PLoS Pathog. 2017. PMID: 28931085 Free PMC article.
References
-
- Bartenschlager R, Lohmann V. Replication of hepatitis C virus. J Gen Virol. 2000;81:1631–1648. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
