A novel inhibitory mechanism of MRTF-A/B on the ICAM-1 gene expression in vascular endothelial cells
- PMID: 26024305
- PMCID: PMC4448521
- DOI: 10.1038/srep10627
A novel inhibitory mechanism of MRTF-A/B on the ICAM-1 gene expression in vascular endothelial cells
Abstract
The roles of myocardin-related transcription factor A (MRTF-A) and MRTF-B in vascular endothelial cells are not completely understood. Here, we found a novel regulatory mechanism for MRTF-A/B function. MRTF-A/B tend to accumulate in the nucleus in arterial endothelial cells in vivo and human aortic endothelial cells (HAoECs) in vitro. In HAoECs, nuclear localization of MRTF-A/B was not significantly affected by Y27632 or latrunculin B, primarily due to the reduced binding of MRTF-A/B to G-actin and in part, to the low level of MRTF-A phosphorylation by ERK. MRTF-A/B downregulation by serum depletion or transfection of siRNA against MRTF-A and/or MRTF-B induced ICAM-1 expression in HAoECs. It is known that nuclear import of nuclear factor-κB (NF-κB) plays a key role in ICAM-1 gene transcription. However, nuclear accumulation of NF-κB p65 was not observed in MRTF-A/B-depleted HAoECs. Our present findings suggest that MRTF-A/B inhibit ICAM-1 mRNA expression by forming a complex with NF-κB p65 in the nucleus. Conversely, downregulation of MRTF-A/B alleviates this negative regulation without further translocation of NF-κB p65 into the nucleus. These results reveal the novel roles of MRTF-A/B in the homeostasis of vascular endothelium.
Figures


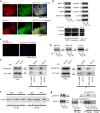



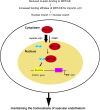
Similar articles
-
Aurintricarboxylic acid inhibits the nuclear factor-κB-dependent expression of intercellular cell adhesion molecule-1 and endothelial cell selectin on activated human endothelial cells.Blood Coagul Fibrinolysis. 2011 Mar;22(2):132-9. doi: 10.1097/MBC.0b013e32834356b6. Blood Coagul Fibrinolysis. 2011. PMID: 21245742
-
Paeonol suppresses intercellular adhesion molecule-1 expression in tumor necrosis factor-alpha-stimulated human umbilical vein endothelial cells by blocking p38, ERK and nuclear factor-kappaB signaling pathways.Int Immunopharmacol. 2007 Mar;7(3):343-50. doi: 10.1016/j.intimp.2006.11.004. Epub 2006 Dec 14. Int Immunopharmacol. 2007. PMID: 17276892
-
Essential role of cofilin-1 in regulating thrombin-induced RelA/p65 nuclear translocation and intercellular adhesion molecule 1 (ICAM-1) expression in endothelial cells.J Biol Chem. 2009 Jul 31;284(31):21047-56. doi: 10.1074/jbc.M109.016444. Epub 2009 May 29. J Biol Chem. 2009. PMID: 19483084 Free PMC article.
-
Docosahexaenoic acid attenuates VCAM-1 expression and NF-κB activation in TNF-α-treated human aortic endothelial cells.J Nutr Biochem. 2011 Feb;22(2):187-94. doi: 10.1016/j.jnutbio.2010.01.007. Epub 2010 Jun 22. J Nutr Biochem. 2011. PMID: 20573493
-
Nucleoskeletal regulation of transcription: Actin on MRTF.Exp Biol Med (Maywood). 2019 Nov;244(15):1372-1381. doi: 10.1177/1535370219854669. Epub 2019 May 29. Exp Biol Med (Maywood). 2019. PMID: 31142145 Free PMC article. Review.
Cited by
-
MKL1 defines the H3K4Me3 landscape for NF-κB dependent inflammatory response.Sci Rep. 2017 Mar 15;7(1):191. doi: 10.1038/s41598-017-00301-w. Sci Rep. 2017. PMID: 28298643 Free PMC article.
-
Genetic Modifiers of the Breast Tumor Microenvironment.Trends Cancer. 2018 Jun;4(6):429-444. doi: 10.1016/j.trecan.2018.04.003. Epub 2018 May 8. Trends Cancer. 2018. PMID: 29860987 Free PMC article. Review.
-
Role of CRP2-MRTF interaction in functions of myofibroblasts.Cell Struct Funct. 2023;48(1):83-98. doi: 10.1247/csf.23004. Cell Struct Funct. 2023. PMID: 37164693 Free PMC article.
-
Nuclear Actin Dynamics in Gene Expression, DNA Repair, and Cancer.Results Probl Cell Differ. 2022;70:625-663. doi: 10.1007/978-3-031-06573-6_23. Results Probl Cell Differ. 2022. PMID: 36348125 Free PMC article.
-
Significance of the p38MAPK-CRP2 axis in myofibroblastic phenotypic transition.Cell Struct Funct. 2023;48(2):199-210. doi: 10.1247/csf.23060. Cell Struct Funct. 2023. PMID: 37899269 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

