NF-κB pathway controls mitochondrial dynamics
- PMID: 26024391
- PMCID: PMC4815975
- DOI: 10.1038/cdd.2015.42
NF-κB pathway controls mitochondrial dynamics
Abstract
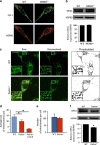
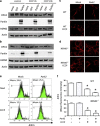
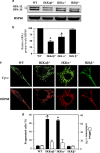
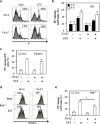
The Optic atrophy 1 protein (OPA1) is a key element in the dynamics and morphology of mitochondria. We demonstrated that the absence of IκB kinase-α, which is a key element of the nonclassical NF-κB pathway, has an impact on the mitochondrial network morphology and OPA1 expression. In contrast, the absence of NF-κB essential modulator (NEMO) or IκB kinase-β, both of which are essential for the canonical NF-κB pathway, has no impact on mitochondrial dynamics. Whereas Parkin has been reported to positively regulate the expression of OPA1 through NEMO, herein we found that PARK2 overexpression did not modify the expression of OPA1. PARK2 expression reduced the levels of Bax, and it prevented stress-induced cell death only in Bak-deficient mouse embryonic fibroblast cells. Collectively, our results point out a role of the nonclassical NF-κB pathway in the regulation of mitochondrial dynamics and OPA1 expression.
Figures




References
-
- Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin-induced mitophagy in the pathogenesis of Parkinson disease. Autophagy 2009; 5: 706–708. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

