A new TLR2 agonist promotes cross-presentation by mouse and human antigen presenting cells
- PMID: 26024409
- PMCID: PMC4635931
- DOI: 10.1080/21645515.2015.1027467
A new TLR2 agonist promotes cross-presentation by mouse and human antigen presenting cells
Abstract
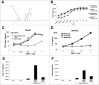
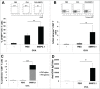
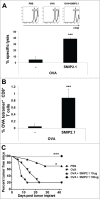
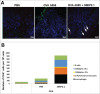
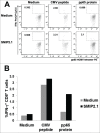
Cross-presentation is the process by which professional APCs load peptides from an extracellularly derived protein onto class I MHC molecules to trigger a CD8(+) T cell response. The ability to enhance this process is therefore relevant for the development of antitumor and antiviral vaccines. We investigated a new TLR2-based adjuvant, Small Molecule Immune Potentiator (SMIP) 2.1, for its ability to stimulate cross-presentation. Using OVA as model antigen, we demonstrated that a SMIP2.1-adjuvanted vaccine formulation induced a greater CD8(+) T cell response, in terms of proliferation, cytokine production and cytolytic activity, than a non-adjuvanted vaccine. Moreover, using an OVA-expressing tumor model, we showed that the CTLs induced by the SMIP2.1 formulated vaccine inhibits tumor growth in vivo. Using a BCR transgenic mouse model we found that B cells could cross-present the OVA antigen when stimulated with SMIP2.1. We also used a flow cytometry assay to detect activation of human CD8(+) T cells isolated from human PBMCs of cytomegalovirus-seropositive donors. Stimulation with SMIP2.1 increased the capacity of human APCs, pulsed in vitro with the pp65 CMV protein, to activate CMV-specific CD8(+) T cells. Therefore, vaccination with an exogenous antigen formulated with SMIP2.1 is a successful strategy for the induction of a cytotoxic T cell response along with antibody production.
Keywords: APC, antigen presenting cell; B cells; BCR, B cell receptor; CMV, cytomegalovirus; CTL, cytotoxic t lymphocyte; DC, dendritic cell; HCMV, human CMV; KO, knock out; LN, lymph node; MHC, major histocompatibility complex; OVA, avalbumin; PBMC, peripheral blood mononuclear cell; SMIP, Small Molecule Immune Potentiator; TLR, toll like receptor; cross presentation/priming; cytotoxic T cells; dendritic cells; vaccination.
Figures


Similar articles
-
A Toll-like receptor 2 agonist-fused antigen enhanced antitumor immunity by increasing antigen presentation and the CD8 memory T cells population.Oncotarget. 2016 May 24;7(21):30804-19. doi: 10.18632/oncotarget.9001. Oncotarget. 2016. PMID: 27127171 Free PMC article.
-
Active CD4+ helper T cells directly stimulate CD8+ cytotoxic T lymphocyte responses in wild-type and MHC II gene knockout C57BL/6 mice and transgenic RIP-mOVA mice expressing islet beta-cell ovalbumin antigen leading to diabetes.Autoimmunity. 2008 Nov;41(7):501-11. doi: 10.1080/08916930802069256. Autoimmunity. 2008. PMID: 18855194
-
PEGylation of a TLR2-agonist-based vaccine delivery system improves antigen trafficking and the magnitude of ensuing antibody and CD8+ T cell responses.Biomaterials. 2017 Aug;137:61-72. doi: 10.1016/j.biomaterials.2017.05.018. Epub 2017 May 11. Biomaterials. 2017. PMID: 28544973
-
Antigen Cross-Presentation and Heat Shock Protein-Based Vaccines.Arch Immunol Ther Exp (Warsz). 2016 Feb;64(1):1-18. doi: 10.1007/s00005-015-0370-x. Epub 2015 Oct 30. Arch Immunol Ther Exp (Warsz). 2016. PMID: 26518489 Review.
-
Adjuvant for vaccine immunotherapy of cancer--focusing on Toll-like receptor 2 and 3 agonists for safely enhancing antitumor immunity.Cancer Sci. 2015 Dec;106(12):1659-68. doi: 10.1111/cas.12824. Epub 2015 Nov 18. Cancer Sci. 2015. PMID: 26395101 Free PMC article. Review.
Cited by
-
A Novel Toll-Like Receptor 2 Agonist Protects Mice in a Prophylactic Treatment Model Against Challenge With Bacillus anthracis.Front Microbiol. 2022 Mar 14;13:803041. doi: 10.3389/fmicb.2022.803041. eCollection 2022. Front Microbiol. 2022. PMID: 35369443 Free PMC article.
-
The Recombinant Profilin from Free-Living Amoebae Induced Allergic Immune Responses via TLR2.J Inflamm Res. 2024 May 13;17:2915-2925. doi: 10.2147/JIR.S450866. eCollection 2024. J Inflamm Res. 2024. PMID: 38764493 Free PMC article.
-
Vaccine-Induced CD8+ T Cell Responses in Children: A Review of Age-Specific Molecular Determinants Contributing to Antigen Cross-Presentation.Front Immunol. 2020 Dec 23;11:607977. doi: 10.3389/fimmu.2020.607977. eCollection 2020. Front Immunol. 2020. PMID: 33424857 Free PMC article. Review.
-
Integrated microbiota-host-metabolome approaches reveal adaptive ruminal changes to prolonged high-grain feeding and phytogenic supplementation in cattle.FEMS Microbiol Ecol. 2024 Jan 24;100(2):fiae006. doi: 10.1093/femsec/fiae006. FEMS Microbiol Ecol. 2024. PMID: 38281064 Free PMC article.
-
Next-Generation Diprovocims with Potent Human and Murine TLR1/TLR2 Agonist Activity That Activate the Innate and Adaptive Immune Response.J Med Chem. 2022 Jul 14;65(13):9230-9252. doi: 10.1021/acs.jmedchem.2c00419. Epub 2022 Jun 29. J Med Chem. 2022. PMID: 35767437 Free PMC article.
References
-
- Wiesel M, Walton S, Richter K, Oxenius A. Virus-specific CD8 T cells: activation, differentiation and memory formation. APMIS 2009; 117:356-81; PMID:19400862; http://dx.doi.org/10.1111/j.1600-0463.2009.02459.x - DOI - PubMed
-
- Bevan MJ. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. The Journal of experimental medicine 1976; 143:1283-8; PMID:1083422; http://dx.doi.org/10.1084/jem.143.5.1283 - DOI - PMC - PubMed
-
- Rock KL, Shen L. Cross-presentation: underlying mechanisms and role in immune surveillance. Immunological reviews 2005; 207:166-83; PMID:16181335; http://dx.doi.org/10.1111/j.0105-2896.2005.00301.x - DOI - PubMed
-
- O'Neill D, Bhardwaj N. Exploiting dendritic cells for active immunotherapy of cancer and chronic infection. Methods Mol Med 2005; 109:1-18; PMID:15585909 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
