Cost-effectiveness of extended-release methylphenidate in children and adolescents with attention-deficit/hyperactivity disorder sub-optimally treated with immediate release methylphenidate
- PMID: 26024479
- PMCID: PMC4449164
- DOI: 10.1371/journal.pone.0127237
Cost-effectiveness of extended-release methylphenidate in children and adolescents with attention-deficit/hyperactivity disorder sub-optimally treated with immediate release methylphenidate
Abstract
Background: Attention-Deficit/Hyperactivity Disorder (ADHD) is a common psychiatric disorder in children and adolescents. Immediate-release methylphenidate (IR-MPH) is the medical treatment of first choice. The necessity to use several IR-MPH tablets per day and associated potential social stigma at school often leads to reduced compliance, sub-optimal treatment, and therefore economic loss. Replacement of IR-MPH with a single-dose extended release (ER-MPH) formulation may improve drug response and economic efficiency.
Objective: To evaluate the cost-effectiveness from a societal perspective of a switch from IR-MPH to ER-MPH in patients who are sub-optimally treated.
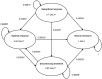
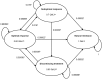
Methods: A daily Markov-cycle model covering a time-span of 10 years was developed including four different health states: (1) optimal response, (2) sub-optimal response, (3) discontinued treatment, and (4) natural remission. ER-MPH options included methylphenidate osmotic release oral system (MPH-OROS) and Equasym XL/Medikinet CR. Both direct costs and indirect costs were included in the analysis, and effects were expressed as quality-adjusted life years (QALYs). Univariate, multivariate as well as probabilistic sensitivity analysis were conducted and the main outcomes were incremental cost-effectiveness ratios.
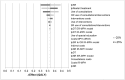
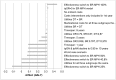
Results: Switching sub-optimally treated patients from IR-MPH to MPH-OROS or Equasym XL/Medikinet CR led to per-patient cost-savings of €4200 and €5400, respectively, over a 10-year treatment span. Sensitivity analysis with plausible variations of input parameters resulted in cost-savings in the vast majority of estimations.
Conclusions: This study lends economic support to switching patients with ADHD with suboptimal response to short-acting IR-MPH to long-acting ER-MPH regimens.
Conflict of interest statement
Figures



Similar articles
-
Long-acting methylphenidate-OROS in youths with attention-deficit hyperactivity disorder suboptimally controlled with immediate-release methylphenidate: a study of cost effectiveness in The Netherlands.CNS Drugs. 2008;22(2):157-70. doi: 10.2165/00023210-200822020-00006. CNS Drugs. 2008. PMID: 18193926
-
Probabilistic Markov Model Estimating Cost Effectiveness of Methylphenidate Osmotic-Release Oral System Versus Immediate-Release Methylphenidate in Children and Adolescents: Which Information is Needed?Pharmacoeconomics. 2015 May;33(5):489-509. doi: 10.1007/s40273-015-0259-x. Pharmacoeconomics. 2015. PMID: 25715975 Free PMC article.
-
Effect of transitioning from extended-release methylphenidate onto osmotic, controlled-release methylphenidate in children/adolescents with ADHD: results of a 3-month non-interventional study.Curr Med Res Opin. 2011;27 Suppl 2:35-44. doi: 10.1185/03007995.2011.601733. Epub 2011 Jul 25. Curr Med Res Opin. 2011. PMID: 21787126 Clinical Trial.
-
A review of OROS methylphenidate (Concerta(®)) in the treatment of attention-deficit/hyperactivity disorder.CNS Drugs. 2014 Nov;28(11):1005-33. doi: 10.1007/s40263-014-0175-1. CNS Drugs. 2014. PMID: 25120227 Review.
-
Osmotic, controlled-release methylphenidate for the treatment of ADHD.Expert Opin Pharmacother. 2006 Oct;7(15):2119-38. doi: 10.1517/14656566.7.15.2119. Expert Opin Pharmacother. 2006. PMID: 17020437 Review.
Cited by
-
Pharmacogenetics of methylphenidate in childhood attention-deficit/hyperactivity disorder: long-term effects.Sci Rep. 2017 Sep 4;7(1):10391. doi: 10.1038/s41598-017-10912-y. Sci Rep. 2017. PMID: 28871191 Free PMC article.
-
Treatment strategies for ADHD: an evidence-based guide to select optimal treatment.Mol Psychiatry. 2019 Mar;24(3):390-408. doi: 10.1038/s41380-018-0116-3. Epub 2018 Jun 28. Mol Psychiatry. 2019. PMID: 29955166 Review.
-
Cost-Effectiveness of Treatments in Children With Attention-Deficit/Hyperactivity Disorder: A Continuous-Time Markov Modeling Approach.MDM Policy Pract. 2019 Aug 17;4(2):2381468319867629. doi: 10.1177/2381468319867629. eCollection 2019 Jul-Dec. MDM Policy Pract. 2019. PMID: 31453364 Free PMC article.
-
Do Costs in the Education Sector Matter? A Systematic Literature Review of the Economic Impact of Psychosocial Problems on the Education Sector.Pharmacoeconomics. 2021 Aug;39(8):889-900. doi: 10.1007/s40273-021-01049-y. Epub 2021 Jun 14. Pharmacoeconomics. 2021. PMID: 34121169 Free PMC article.
-
Social adjustment and family function after drug switch from IR-methylphenidate to OROS-methylphenidate in patients with attention-deficit/hyperactivity disorder.Neuropsychiatr Dis Treat. 2018 Oct 23;14:2783-2791. doi: 10.2147/NDT.S176913. eCollection 2018. Neuropsychiatr Dis Treat. 2018. PMID: 30425496 Free PMC article.
References
-
- Faber A, van Agthoven M, Kalverdijk LJ, Tobi H, de Jong-van den Berg LTW, Annemans L, et al. Long-acting methylphenidate-OROS in youths with attention-deficit hyperactivity disorder suboptimally controlled with immediate-release methylphenidate: a study of cost effectiveness in the Netherlands. CNS Drugs. 2008; 22 (2): 157–170. - PubMed
-
- Polanczyk G, Rohde LA. Epidemiology of attention- deficit/hyperactivity disorder across the lifespan. Curr Opin Psychiatry. 2007; 20(4):386–92. - PubMed
-
- National Institute for Health and Care Excellence. Diagnosis and management of ADHD in children, young people and adults (National Clinical Practice Number 72). 2008; Retrieved from http://www.nice.org.uk/CG72 Accessed 8 July 2014
-
- Jensen PS, Garcia JA, Glied S, Crowe M, Foster M, Schlander M, et al. Cost-effectiveness of ADHD treatments: findings from the multimodal treatment study of children with ADHD. Am J Psychiatry. 2005; 162(9):1628–36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

