Cell kinetic studies fail to identify sequentially proliferating progenitors as the major source of epithelial renewal in the adult murine prostate
- PMID: 26024527
- PMCID: PMC4449166
- DOI: 10.1371/journal.pone.0128489
Cell kinetic studies fail to identify sequentially proliferating progenitors as the major source of epithelial renewal in the adult murine prostate
Abstract
There is evidence that stem cells and their progeny play a role in the development of the prostate. Although stem cells are also considered to give rise to differentiated progeny in the adult prostate epithelium ex vivo, the cohort of adult prostate stem cells in vivo as well as the mechanisms by which the adult prostate epithelium is maintained and regenerated remain highly controversial. We have attempted to resolve this conundrum by performing in vivo tracing of serially replicating cells after the sequential administration of two thymidine analogues to mice. Our results show that, during normal prostate homeostasis, sequentially proliferating cells are detected at a rate that is consistent with a stochastic process. These findings indicate that in vivo, under steady-state conditions, most adult prostate epithelial cells do not represent the progeny of a small number of specialized progenitors that generate sequentially replicating transit-amplifying (TA) cells but are formed by stochastic cell division. Similarly, no rapidly cycling TA cells were detected during regeneration following one cycle of androgen-mediated involution/regeneration of the prostate epithelium. These findings greatly enhance our understanding of the mechanisms regulating prostate epithelial cell renewal and may have significant implications in defining the cell of origin of proliferative prostatic diseases.
Conflict of interest statement
Figures
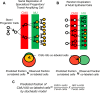
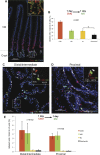

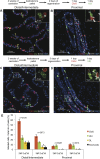
References
-
- Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. - PubMed
-
- Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell. 2007;12:817–826. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

