Mio acts in the Drosophila brain to control nutrient storage and feeding
- PMID: 26024590
- PMCID: PMC4470767
- DOI: 10.1016/j.gene.2015.05.055
Mio acts in the Drosophila brain to control nutrient storage and feeding
Abstract
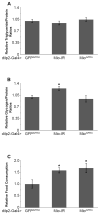
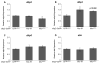
Animals recognize the availability of nutrients and regulate the intake and storage of these nutrients accordingly. However, the molecular mechanisms underlying nutrient sensing and subsequent changes in behavior and metabolism are not fully understood. Mlx interactor (Mio), the Drosophila homolog of carbohydrate response element binding protein (ChREBP), functions as a transcription factor in the fat body of the fly to control triglyceride storage as well as feeding, suggesting that Mio may act in a nutrient-sensing pathway to coordinate food consumption and metabolism. Here, we show that Mio functions in neurons in Drosophila to regulate feeding and nutrient storage. Pan-neuronal disruption of Mio function leads to increased triglyceride and glycogen storage, and this phenotype is not due to increased food consumption. Interestingly, targeted disruption of Mio specifically in the insulin-producing cells (IPCs) has little effect on nutrient storage, but increases food consumption suggesting that Mio acts in these neurons to control feeding behavior. Since Mio is a transcription factor, one possible way Mio may act in the IPCs to control feeding is through regulating the expression of Drosophila insulin-like peptides (dilps) or drosulfakinin (dsk), neuropeptides produced in the IPCs. Consistent with this hypothesis, IPC-specific knockdown of Mio leads to an increase in dilp3 expression, while not affecting dilp2, 5 or dsk levels. Together, this study indicates a new function for Mio in the Drosophila brain and specifically in the IPCs, controlling neuropeptide gene expression, feeding and metabolism in accordance with nutrient availability.
Keywords: Brain; Drosophila; Feeding; Insulin-like peptide; Metabolism.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Mio/dChREBP coordinately increases fat mass by regulating lipid synthesis and feeding behavior in Drosophila.Biochem Biophys Res Commun. 2012 Sep 14;426(1):43-8. doi: 10.1016/j.bbrc.2012.08.028. Epub 2012 Aug 12. Biochem Biophys Res Commun. 2012. PMID: 22910416 Free PMC article.
-
The Regulation of Muscle Structure and Metabolism by Mio/dChREBP in Drosophila.PLoS One. 2015 Aug 25;10(8):e0136504. doi: 10.1371/journal.pone.0136504. eCollection 2015. PLoS One. 2015. PMID: 26305467 Free PMC article.
-
Drosophila insulin-producing cells are differentially modulated by serotonin and octopamine receptors and affect social behavior.PLoS One. 2014 Jun 12;9(6):e99732. doi: 10.1371/journal.pone.0099732. eCollection 2014. PLoS One. 2014. PMID: 24923784 Free PMC article.
-
Factors that regulate expression patterns of insulin-like peptides and their association with physiological and metabolic traits in Drosophila.Insect Biochem Mol Biol. 2021 Aug;135:103609. doi: 10.1016/j.ibmb.2021.103609. Epub 2021 Jun 17. Insect Biochem Mol Biol. 2021. PMID: 34146686 Review.
-
Functional implications of Drosophila insulin-like peptides in metabolism, aging, and dietary restriction.Front Physiol. 2013 Oct 16;4:288. doi: 10.3389/fphys.2013.00288. Front Physiol. 2013. PMID: 24137131 Free PMC article. Review.
Cited by
-
Insulin/IGF signaling in Drosophila and other insects: factors that regulate production, release and post-release action of the insulin-like peptides.Cell Mol Life Sci. 2016 Jan;73(2):271-90. doi: 10.1007/s00018-015-2063-3. Epub 2015 Oct 15. Cell Mol Life Sci. 2016. PMID: 26472340 Free PMC article. Review.
-
Glut1 Functions in Insulin-Producing Neurons to Regulate Lipid and Carbohydrate Storage in Drosophila.Biomolecules. 2024 Aug 20;14(8):1037. doi: 10.3390/biom14081037. Biomolecules. 2024. PMID: 39199423 Free PMC article.
-
Using Drosophila to discover mechanisms underlying type 2 diabetes.Dis Model Mech. 2016 Apr;9(4):365-76. doi: 10.1242/dmm.023887. Dis Model Mech. 2016. PMID: 27053133 Free PMC article. Review.
-
Modeling dietary influences on offspring metabolic programming in Drosophila melanogaster.Reproduction. 2016 Sep;152(3):R79-90. doi: 10.1530/REP-15-0595. Reproduction. 2016. PMID: 27450801 Free PMC article. Review.
-
Regulation of Carbohydrate Energy Metabolism in Drosophila melanogaster.Genetics. 2017 Dec;207(4):1231-1253. doi: 10.1534/genetics.117.199885. Genetics. 2017. PMID: 29203701 Free PMC article. Review.
References
-
- Suzuki K, Simpson KA, Minnion JS, Shillito JC, Bloom SR. The role of gut hormones and the hypothalamus in appetite regulation. Endocr J. 2010;57:359–372. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

