Chemically Programmed Bispecific Antibody Targeting Legumain Protease and αvβ3 Integrin Mediates Strong Antitumor Effects
- PMID: 26024761
- PMCID: PMC8341100
- DOI: 10.1021/acs.molpharmaceut.5b00257
Chemically Programmed Bispecific Antibody Targeting Legumain Protease and αvβ3 Integrin Mediates Strong Antitumor Effects
Abstract
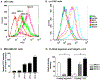
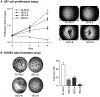
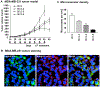
A chemically programmed bispecific antibody (cp-bsAb) that targeted cysteine protease legumain and αvβ3 integrin has been prepared using the aldolase antibody chemical programming (AACP) strategy. In vitro evaluation of the anti-legumain, anti-integrin cp-bsAb and its comparison with cpAbs targeting either integrin or legumain have shown that the former possesses superior functions, including receptor binding and inhibitory effects on cell proliferation as well as capillary tube formation, among all three cpAbs. The anti-legumain, anti-integrin cp-bsAb also inhibited growth of primary tumor more effectively than either anti-legumain or anti-integrin cpAb as observed in the MDA-MB-231 human breast cancer mouse model. The AACP-based cp-bsAb, which contains a generic aldolase antibody, can also serve as a suitable platform for combination therapy, where two equally potent compounds are used to target extracellular receptors.
Keywords: 38C2; aldolase; alpha(v)beta(3); antibody; bispecific; chemical; integrin; legumain; protease.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Multiple catalytic aldolase antibodies suitable for chemical programming.Bioorg Med Chem Lett. 2009 Jul 15;19(14):3821-4. doi: 10.1016/j.bmcl.2009.04.041. Epub 2009 Apr 18. Bioorg Med Chem Lett. 2009. PMID: 19428247 Free PMC article.
-
Targeting cell surface alpha(v)beta(3) integrin increases therapeutic efficacies of a legumain protease-activated auristatin prodrug.Mol Pharm. 2012 Jan 1;9(1):168-75. doi: 10.1021/mp200434n. Epub 2011 Nov 22. Mol Pharm. 2012. PMID: 22044266 Free PMC article.
-
Chemically programmed antibodies targeting multiple alpha(v) integrins and their effects on tumor-related functions in vitro.Bioconjug Chem. 2011 Aug 17;22(8):1535-44. doi: 10.1021/bc2000879. Epub 2011 Aug 1. Bioconjug Chem. 2011. PMID: 21774545 Free PMC article.
-
The Mammalian Cysteine Protease Legumain in Health and Disease.Int J Mol Sci. 2022 Dec 15;23(24):15983. doi: 10.3390/ijms232415983. Int J Mol Sci. 2022. PMID: 36555634 Free PMC article. Review.
-
Mammalian legumain - A lysosomal cysteine protease with extracellular functions?Biochimie. 2019 Nov;166:77-83. doi: 10.1016/j.biochi.2019.06.002. Epub 2019 Jun 7. Biochimie. 2019. PMID: 31181234 Review.
Cited by
-
The Effect of TNF and VEGF on the Properties of Ea.hy926 Endothelial Cells in a Model of Multi-Cellular Spheroids.Acta Naturae. 2018 Jan-Mar;10(1):34-42. Acta Naturae. 2018. PMID: 29713517 Free PMC article.
-
Chemically Programmed Bispecific Antibodies in Diabody Format.J Biol Chem. 2016 Sep 9;291(37):19661-73. doi: 10.1074/jbc.M116.745588. Epub 2016 Jul 21. J Biol Chem. 2016. PMID: 27445334 Free PMC article.
-
SMI-Ribosome inactivating protein conjugates selectively inhibit tumor cell growth.Chem Commun (Camb). 2017 Apr 11;53(30):4234-4237. doi: 10.1039/c7cc00745k. Chem Commun (Camb). 2017. PMID: 28357420 Free PMC article.
-
Platelet microparticle-mediated transfer of miR-939 to epithelial ovarian cancer cells promotes epithelial to mesenchymal transition.Oncotarget. 2017 Oct 27;8(57):97464-97475. doi: 10.18632/oncotarget.22136. eCollection 2017 Nov 14. Oncotarget. 2017. PMID: 29228624 Free PMC article.
-
Novel transgenic pigs with enhanced growth and reduced environmental impact.Elife. 2018 May 22;7:e34286. doi: 10.7554/eLife.34286. Elife. 2018. PMID: 29784082 Free PMC article.
References
-
- Chen JM; Dando PM; Rawlings ND; Brown MA; Young NE; Stevens RA; Hewitt E; Watts C; Barrett AJ Cloning, isolation, and characterization of mammalian legumain, an asparaginyl endopeptidase. J. Biol. Chem 1997, 272, 8090–98. - PubMed
-
- Shirahama-Noda K; Yamamoto A; Sugihara K; Hashimoto N; Asano M; Nishimura M; Hara-Nishimura I Biosynthetic processing of cathepsins and lysosomal degradation are abolished in asparaginyl endopeptidase-deficient mice. J. Biol. Chem 2003, 278, 33194–9. - PubMed
-
- Chen JM; Fortunato M; Stevens RA; Barrett AJ Activation of progelatinase A by mammalian legumain, a recently discovered cysteine proteinase. Biol. Chem 2001, 382, 777–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

