Transient mitochondrial permeability transition mediates excitotoxicity in glutamate-sensitive NSC34D motor neuron-like cells
- PMID: 26024861
- PMCID: PMC4586369
- DOI: 10.1016/j.expneurol.2015.05.010
Transient mitochondrial permeability transition mediates excitotoxicity in glutamate-sensitive NSC34D motor neuron-like cells
Abstract
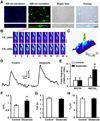
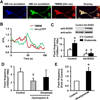
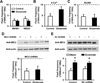
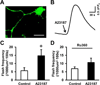
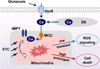
Excitotoxicity plays a critical role in neurodegenerative disease. Cytosolic calcium overload and mitochondrial dysfunction are among the major mediators of high level glutamate-induced neuron death. Here, we show that the transient opening of mitochondrial permeability transition pore (tMPT) bridges cytosolic calcium signaling and mitochondrial dysfunction and mediates glutamate-induced neuron death. Incubation of the differentiated motor neuron-like NSC34D cells with glutamate (1mM) acutely induces cytosolic calcium transient (30% increase). Glutamate also stimulates tMPT opening, as reflected by a 2-fold increase in the frequency of superoxide flash, a bursting superoxide production event in individual mitochondria coupled to tMPT opening. The glutamate-induced tMPT opening is attenuated by suppressing cytosolic calcium influx and abolished by inhibiting mitochondrial calcium uniporter (MCU) with Ru360 (100 μM) or MCU shRNA. Further, increased cytosolic calcium is sufficient to induce tMPT in a mitochondrial calcium dependent manner. Finally, chronic glutamate incubation (24h) persistently elevates the probability of tMPT opening, promotes oxidative stress and induces neuron death. Attenuating tMPT activity or inhibiting MCU protects NSC34D cells from glutamate-induced cell death. These results indicate that high level glutamate-induced neuron toxicity is mediated by tMPT, which connects increased cytosolic calcium signal to mitochondrial dysfunction.
Keywords: Excitotoxicity; Glutamate; Mitochondrial calcium uniporter; Neuron death; Superoxide flashes; Transient mitochondrial permeability transition.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
N-methyl-D-aspartate receptor-mediated mitochondrial Ca(2+) overload in acute excitotoxic motor neuron death: a mechanism distinct from chronic neurotoxicity after Ca(2+) influx.J Neurosci Res. 2001 Mar 1;63(5):377-87. doi: 10.1002/1097-4547(20010301)63:5<377::AID-JNR1032>3.0.CO;2-#. J Neurosci Res. 2001. PMID: 11223912
-
AMPA receptor activation causes preferential mitochondrial Ca²⁺ load and oxidative stress in motor neurons.Brain Res. 2015 Aug 7;1616:1-9. doi: 10.1016/j.brainres.2015.04.042. Epub 2015 May 2. Brain Res. 2015. PMID: 25944722
-
Glutamate excitotoxicity and Ca2+-regulation of respiration: Role of the Ca2+ activated mitochondrial transporters (CaMCs).Biochim Biophys Acta. 2016 Aug;1857(8):1158-1166. doi: 10.1016/j.bbabio.2016.04.003. Epub 2016 Apr 7. Biochim Biophys Acta. 2016. PMID: 27060251 Review.
-
Calcium-induced cardiac mitochondrial dysfunction is predominantly mediated by cyclosporine A-dependent mitochondrial permeability transition pore.Arch Med Res. 2012 Jul;43(5):333-8. doi: 10.1016/j.arcmed.2012.06.010. Epub 2012 Jul 21. Arch Med Res. 2012. PMID: 22824212
-
Excitotoxicity and mitochondria.Biochem Soc Symp. 1999;66:55-67. doi: 10.1042/bss0660055. Biochem Soc Symp. 1999. PMID: 10989657 Review.
Cited by
-
Bacopa monnieri attenuates glutamate-induced nociception and brain mitochondrial toxicity in Zebrafish.Metab Brain Dis. 2022 Feb;37(2):383-396. doi: 10.1007/s11011-021-00874-6. Epub 2021 Nov 24. Metab Brain Dis. 2022. Retraction in: Metab Brain Dis. 2023 Oct;38(7):2503. doi: 10.1007/s11011-023-01284-6. PMID: 34817757 Retracted.
-
The mitochondrial Ca2+ uniporter: regulation by auxiliary subunits and signal transduction pathways.Am J Physiol Cell Physiol. 2016 Jul 1;311(1):C67-80. doi: 10.1152/ajpcell.00319.2015. Epub 2016 Apr 27. Am J Physiol Cell Physiol. 2016. PMID: 27122161 Free PMC article. Review.
-
Glial Cell AMPA Receptors in Nervous System Health, Injury and Disease.Int J Mol Sci. 2019 May 17;20(10):2450. doi: 10.3390/ijms20102450. Int J Mol Sci. 2019. PMID: 31108947 Free PMC article. Review.
-
Mitochondria Initiate and Regulate Sarcopenia.Exerc Sport Sci Rev. 2017 Apr;45(2):58-69. doi: 10.1249/JES.0000000000000101. Exerc Sport Sci Rev. 2017. PMID: 28098577 Free PMC article. Review.
-
NSC-34 Motor Neuron-Like Cells Are Unsuitable as Experimental Model for Glutamate-Mediated Excitotoxicity.Front Cell Neurosci. 2016 May 9;10:118. doi: 10.3389/fncel.2016.00118. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27242431 Free PMC article.
References
-
- Al-Chalabi A, Leigh PN. Recent advances in amyotrophic lateral sclerosis. Curr Opin Neurol. 2000;13:397–405. - PubMed
-
- Azbill RD, Mu X, Springer JE. Riluzole increases high-affinity glutamate uptake in rat spinal cord synaptosomes. Brain Res. 2000;871:175–180. - PubMed
-
- Berry JD, Shefner JM, Conwit R, Schoenfeld D, Keroack M, Felsenstein D, Krivickas L, David WS, Vriesendorp F, Pestronk A, Caress JB, Katz J, Simpson E, Rosenfeld J, Pascuzzi R, Glass J, Rezania K, Rothstein JD, Greenblatt DJ, Cudkowicz ME. Design and initial results of a multi-phase randomized trial of ceftriaxone in amyotrophic lateral sclerosis. PLoS One. 2013;8:e61177. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

