Cyclic stretch stimulates mitochondrial reactive oxygen species and Nox4 signaling in pulmonary artery smooth muscle cells
- PMID: 26024892
- PMCID: PMC4504971
- DOI: 10.1152/ajplung.00097.2014
Cyclic stretch stimulates mitochondrial reactive oxygen species and Nox4 signaling in pulmonary artery smooth muscle cells
Abstract
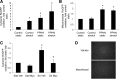
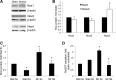
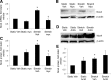
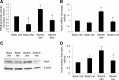
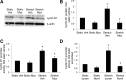
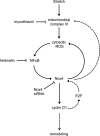
This study was designed to determine whether cyclic stretch induces a persistent pulmonary hypertension of the newborn (PPHN) phenotype of increased NADPH oxidase (Nox) 4 signaling in control pulmonary artery smooth muscle cells (PASMC), and to identify the signal transduction molecules involved. To achieve this, PPHN was induced in lambs by antenatal ligation of the ductus arteriosus at 128 days gestation. After 9 days, lungs and PASMC were isolated from control (twin) and PPHN lambs. Control PASMC were exposed to cyclic stretch at 1 Hz and 15% elongation for 24 h. Stretch-induced Nox4 expression was attenuated by inhibition of mitochondrial complex III and NF-κB, and stretch-induced protein thiol oxidation was attenuated by Nox4 small interfering RNA and complex III inhibition. NF-κB activity was increased by stretch in a complex III-dependent fashion, and stretch-induced cyclin D1 expression was attenuated by complex III inhibition and Nox4 small interfering RNA. This is the first study to show that cyclic stretch increases Nox4 expression via mitochondrial complex III-induced activation of NF-κB in fetal PASMC, resulting in ROS signaling and increased cyclin D1 expression. Targeting these signaling molecules may attenuate pulmonary vascular remodeling associated with PPHN.
Keywords: NADPH oxidase; pulmonary hypertension; reactive oxygen species.
Copyright © 2015 the American Physiological Society.
Figures
References
-
- Abman SH, Accurso FJ. Acute effects of partial compression of ductus arteriosus on fetal pulmonary circulation. Am J Physiol Heart Circ Physiol 257: H626–H634, 1989. - PubMed
-
- Ali M, Mungai P, Schumacker P. Stretch-induced phosphorylation of focal adhesion kinase in endothelial cells:role of mitochondrial oxidants. Am J Physiol Lung Cell Mol Physiol 291: L38–L45, 2006. - PubMed
-
- Ali M, Pearlstein D, Mathieu C, Schumacker P. Mitochondrial requirement for endothelial responses to cyclic strain: implications for mechanotransduction. Am J Physiol Lung Cell Mol Physiol 287: L486–L496, 2004. - PubMed
-
- Ambasta R, Kumar P, Griendling K, Schmidt H, Busse R, Brandes R. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem 279: 45935–45941, 2004. - PubMed
-
- Aoyama T, Paik YH, Watanabe S, Laleu B, Gaggini F, Fioraso-Cartier L, Molango S, Heitz F, Merlot C, Szyndralewiez C, Page P, Brenner DA. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology 56: 2316–2327, 2012. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

