State of the art of leadless pacing
- PMID: 26024918
- PMCID: PMC4617371
- DOI: 10.1093/europace/euv096
State of the art of leadless pacing
Abstract
Despite undisputable benefits, conventional pacemaker therapy is associated with specific complications related to the subcutaneous device and the transvenous leads. Recently, two miniaturized leadless pacemakers, Nanostim™ (St. Jude Medical) and Micra™ (Medtronic), which can be completely implanted inside the right ventricle using steerable delivery systems, entered clinical application. The WiCS™-cardiac resynchronisation therapy (CRT) system (wireless cardiac stimulation for CRT, EBR Systems) delivers leadless left ventricular endocardial stimulation for cardiac resynchronization. In addition to obvious cosmetic benefits, leadless pacing systems may have the potential to overcome some complications of conventional pacing. However, acute and long-term complications still remains to be determined, as well as the feasibility of device explantation years after device placement.
Keywords: Catheter delivery; Leadless pacing; Pacemaker.
© The Author 2015. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures


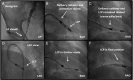
Comment in
-
An important advantage of the leadless pacemakers: magnetic resonance imaging compatibility.Europace. 2016 Apr;18(4):628-9. doi: 10.1093/europace/euv246. Epub 2016 Feb 6. Europace. 2016. PMID: 26851812 No abstract available.
References
-
- Spickler JW, Rasor NS, Kezdi P, Misra SN, Robins KE, LeBoeuf C. Totally self-contained intracardiac pacemaker. J Electrocardiol 1970;3:325–31. - PubMed
-
- Vardas PE, Politopoulos E, Manios E, Parthenakis F, Tsagarakis C. A miniature pacemaker introduced intravenously and implanted endocardially. Preliminary findings form an experimental study. Eur J CPE 1991;1:27–30.
-
- Echt DS, Cowan MW, Riley RE, Brisken AF. Feasibility and safety of a novel technology for pacing without leads. Heart Rhythm 2006;3:1202–6. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

