Measures of follow-up in time-to-event studies: Why provide them and what should they be?
- PMID: 26025565
- PMCID: PMC4506242
- DOI: 10.1177/1740774515586176
Measures of follow-up in time-to-event studies: Why provide them and what should they be?
Abstract
Background/aims: There is some consensus among authors of reports of clinical studies that a measure of follow-up time is informative for the interpretation of the Kaplan-Meier estimate of the survivor function of the event time of interest. Previous authors have suggested that length of follow-up is important to report because the findings of a study should be extracted from the time frame in which most of the subjects have had the event or have remained under observation. This time frame is where the Kaplan-Meier estimate is most stable. This concept of stability is relative to the potential maximum information about the event time distribution contained in the sample; it is not relative to the true, population survivor function. A measure of stability is useful for the interpretation of an interim analysis in which an immature survivor function is presented. Our interest in this article lies in characterizing the unobserved, complete follow-up Kaplan-Meier estimate based on the observed, partial follow-up estimate. Our focus is not on characterizing the true event time distribution relative to its estimate. The concept of stability has not been well-defined in the literature, which has led to inconsistency and lack of transparency across trials in their attempts to capture it through a variety of measures of follow-up.
Methods: We report the results of a survey of recent literature on cancer clinical trials and summarize whether follow-up is reported and if so, whether it is well-defined. We define commonly used measures of follow-up in clinical studies.
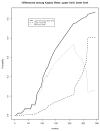
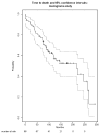
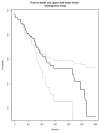
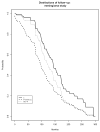
Results: We explain how each measure should be assessed to evaluate the stability of the Kaplan-Meier estimate for the event, and we identify relationships among measures. We propose a new measure that better conveys the desired information about the stability of the current Kaplan-Meier estimate relative to one based on complete follow-up. We apply the proposed measure to a meningioma study for illustration.
Conclusion: It is useful for reports of clinical studies to supplement Kaplan-Meier estimates with quantitative assessments of the stability of those estimates relative to the potential follow-up of study participants. We justify the use of one commonly used measure, and we propose a new measure that most directly accomplishes this goal.
Keywords: Censoring; clinical trials; observation time.
© The Author(s) 2015.
Conflict of interest statement
None declared.
Figures
Similar articles
-
Kaplan-Meier curves for survivor causal effects with time-to-event outcomes.Clin Trials. 2013 Aug;10(4):515-21. doi: 10.1177/1740774513483601. Epub 2013 Apr 22. Clin Trials. 2013. PMID: 23610455 Clinical Trial.
-
Evaluating protocol lifecycle time intervals in HIV/AIDS clinical trials.Clin Trials. 2014 Oct;11(5):553-9. doi: 10.1177/1740774514540814. Epub 2014 Jun 30. Clin Trials. 2014. PMID: 24980279 Free PMC article.
-
A multiple imputation method for sensitivity analyses of time-to-event data with possibly informative censoring.J Biopharm Stat. 2014;24(2):229-53. doi: 10.1080/10543406.2013.860769. J Biopharm Stat. 2014. PMID: 24605967 Free PMC article.
-
Methods to Analyse Time-to-Event Data: The Kaplan-Meier Survival Curve.Oxid Med Cell Longev. 2021 Sep 20;2021:2290120. doi: 10.1155/2021/2290120. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34594473 Free PMC article. Review.
-
Reporting of loss to follow-up information in randomised controlled trials with time-to-event outcomes: a literature survey.BMC Med Res Methodol. 2011 Sep 21;11:130. doi: 10.1186/1471-2288-11-130. BMC Med Res Methodol. 2011. PMID: 21936924 Free PMC article. Review.
Cited by
-
Adverse Impact of DNA Methylation Regulatory Gene Mutations on the Prognosis of AML Patients in the 2017 ELN Favorable Risk Group, Particularly Those Defined by NPM1 Mutation.Diagnostics (Basel). 2021 May 29;11(6):986. doi: 10.3390/diagnostics11060986. Diagnostics (Basel). 2021. PMID: 34072516 Free PMC article.
-
Glioblastoma in young adult patients: contemporary patterns of care and survival in the United States.J Neurooncol. 2025 Jul 15. doi: 10.1007/s11060-025-05135-5. Online ahead of print. J Neurooncol. 2025. PMID: 40663309 No abstract available.
-
Replication of Overall Survival, Progression-Free Survival, and Overall Response in Chemotherapy Arms of Non-Small Cell Lung Cancer Trials Using Real-World Data.Clin Cancer Res. 2022 Jul 1;28(13):2844-2853. doi: 10.1158/1078-0432.CCR-22-0471. Clin Cancer Res. 2022. PMID: 35511917 Free PMC article.
-
Incident thrombocytopenia and bleeding risk in elderly patients with atrial fibrillation on direct oral anticoagulants: insights from the ATHEROsclerosis in Atrial Fibrillation study.Res Pract Thromb Haemost. 2024 Sep 23;8(7):102575. doi: 10.1016/j.rpth.2024.102575. eCollection 2024 Oct. Res Pract Thromb Haemost. 2024. PMID: 39822327 Free PMC article.
-
An epidemic of chikungunya in northwestern Bangladesh in 2011.PLoS One. 2019 Mar 11;14(3):e0212218. doi: 10.1371/journal.pone.0212218. eCollection 2019. PLoS One. 2019. PMID: 30856200 Free PMC article.
References
-
- Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. - PubMed
-
- Korn EL. Censoring distributions as a measure of follow-up in survival analysis. Stat Med. 1986;5:255–260. - PubMed
-
- Shuster JJ. Median follow-up in clinical trials. J Clin Oncol. 1991;9:191–192. - PubMed
-
- Green S, Benedetti J, Smith A, et al. Chapman & Hall/CRC Interdisciplinary Statistics Series. 3. Boca Raton, FL: CRC Press; 2012. Clinical Trials in Oncology; pp. 155–159.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources