CONSORT in China: past development and future direction
- PMID: 26026311
- PMCID: PMC4467063
- DOI: 10.1186/s13063-015-0769-z
CONSORT in China: past development and future direction
Abstract
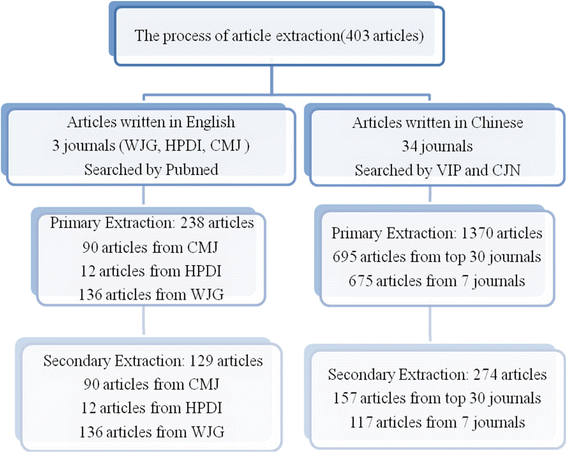
The Consolidated Standards of Reporting Trials (CONSORT) Statement was published in 1996, and first introduced to China in 2001. Although CONSORT has been widely accepted in high-quality international journals, we still need to have more investigation on how many Chinese journals have adopted the CONSORT Statement, and whether the quality of reporting has improved. A systematic search of the "Instructions to authors" in all Chinese medical journals in China Academic Journals (CAJ) Full-text Database was conducted up to February 2012 and only 7 journals officially listed the requirements of the CONSORT Statement. The research articles about randomized controlled trials (RCTs) published in 2002, 2004, 2006, 2008, and 2010 from journals which had specifically adopted the CONSORT Statement, and from 30 top journals based on the Chinese Science Citation Index (CSCI) 2011 as the control group, were identified. The quality of both cohorts of articles was assessed using the revised CONSORT Checklist and Jadad scale. A total of 1221 Chinese medical journals was identified. Only seven journals stated clearly in the "Instructions to authors" that authors should adopt the CONSORT requirement in the clinical trial paper. None of these journals is among the control group in the CSCI 2011. In the selected years, a total of 171 articles from 7 journals which had adopted CONSORT and 232 articles in the control were identified as including RCT trials. The average scores according to the revised CONSORT Checklist were 29.47 for the CONSORT-adopting journals and 25.57 for the control group; while the average scores based on the Jadad scale were 2.53 for CONSORT-adopting journals and 1.97 for the control group. Few journals among Chinese medical journals have adopted the CONSORT Statement. The overall quality of RCT reports in the 7 journals which have adopted CONSORT was better than those in the top 30 journals which have not adopted CONSORT. The quality of RCT reports in Chinese journals needs further improvement, and the CONSORT Statement could be a very helpful guideline.
Figures


Similar articles
-
Has the quality of abstracts for randomised controlled trials improved since the release of Consolidated Standards of Reporting Trial guideline for abstract reporting? A survey of four high-profile anaesthesia journals.Eur J Anaesthesiol. 2011 Jul;28(7):485-92. doi: 10.1097/EJA.0b013e32833fb96f. Eur J Anaesthesiol. 2011. PMID: 21037480
-
Chinese authors do need CONSORT: reporting quality assessment for five leading Chinese medical journals.Contemp Clin Trials. 2008 Sep;29(5):727-31. doi: 10.1016/j.cct.2008.05.003. Epub 2008 May 18. Contemp Clin Trials. 2008. PMID: 18579449
-
Does the CONSORT checklist for abstracts improve the quality of reports of randomized controlled trials on clinical pathways?J Eval Clin Pract. 2014 Dec;20(6):827-33. doi: 10.1111/jep.12200. Epub 2014 Jun 11. J Eval Clin Pract. 2014. PMID: 24916891
-
Does the reporting of randomized clinical trials published in Chinese pediatrics journals improve after the CONSORT Statement is adopted?Contemp Clin Trials. 2012 Sep;33(5):889-94. doi: 10.1016/j.cct.2012.06.008. Epub 2012 Jul 6. Contemp Clin Trials. 2012. PMID: 22765929 Review.
-
The quality of reporting of randomized controlled trials of traditional Chinese medicine: a survey of 13 randomly selected journals from mainland China.Clin Ther. 2007 Jul;29(7):1456-67. doi: 10.1016/j.clinthera.2007.07.023. Clin Ther. 2007. PMID: 17825697 Review.
Cited by
-
Effectiveness of acupuncture for cancer pain: protocol for an umbrella review and meta-analyses of controlled trials.BMJ Open. 2017 Dec 10;7(12):e018494. doi: 10.1136/bmjopen-2017-018494. BMJ Open. 2017. PMID: 29229658 Free PMC article.
-
Systematic review and meta-analyses of studies analysing instructions to authors from 1987 to 2017.Nat Commun. 2021 Oct 5;12(1):5840. doi: 10.1038/s41467-021-26027-y. Nat Commun. 2021. PMID: 34611157 Free PMC article.
-
Quality of reporting and risk of bias: a review of randomised trials in occupational health.Occup Environ Med. 2021 Sep;78(9):691-696. doi: 10.1136/oemed-2020-107038. Epub 2021 Jun 23. Occup Environ Med. 2021. PMID: 34162718 Free PMC article.
-
STROBE, CONSORT, PRISMA, MOOSE, STARD, SPIRIT, and other guidelines - Overview and application.Saudi J Anaesth. 2024 Jan-Mar;18(1):137-141. doi: 10.4103/sja.sja_545_23. Epub 2024 Jan 2. Saudi J Anaesth. 2024. PMID: 38313708 Free PMC article.
-
The history of controlled clinical trials in China. Part 2: from the advent of large-scale multicentre randomised controlled trials through contemporary self-reflection.J R Soc Med. 2025 Jun 3:1410768251347259. doi: 10.1177/01410768251347259. Online ahead of print. J R Soc Med. 2025. PMID: 40459228 Free PMC article. No abstract available.
References
-
- Moher D. CONSORT: how CONSORT began. http://www.consort-statement.org/about-consort/history. Accessed on Jan. 12nd, 2015.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

