A Corticostriatal Path Targeting Striosomes Controls Decision-Making under Conflict
- PMID: 26027737
- PMCID: PMC4477966
- DOI: 10.1016/j.cell.2015.04.049
A Corticostriatal Path Targeting Striosomes Controls Decision-Making under Conflict
Abstract
A striking neurochemical form of compartmentalization has been found in the striatum of humans and other species, dividing it into striosomes and matrix. The function of this organization has been unclear, but the anatomical connections of striosomes indicate their relation to emotion-related brain regions, including the medial prefrontal cortex. We capitalized on this fact by combining pathway-specific optogenetics and electrophysiology in behaving rats to search for selective functions of striosomes. We demonstrate that a medial prefronto-striosomal circuit is selectively active in and causally necessary for cost-benefit decision-making under approach-avoidance conflict conditions known to evoke anxiety in humans. We show that this circuit has unique dynamic properties likely reflecting striatal interneuron function. These findings demonstrate that cognitive and emotion-related functions are, like sensory-motor processing, subject to encoding within compartmentally organized representations in the forebrain and suggest that striosome-targeting corticostriatal circuits can underlie neural processing of decisions fundamental for survival.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

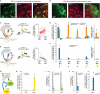

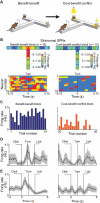
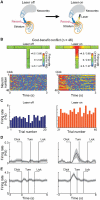


Comment in
-
What's Worth the Risk? A Neural Circuit for Trade-Offs.Cell. 2015 Jun 4;161(6):1243-4. doi: 10.1016/j.cell.2015.05.031. Cell. 2015. PMID: 26046432
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

