A TRP Channel Senses Lysosome Neutralization by Pathogens to Trigger Their Expulsion
- PMID: 26027738
- PMCID: PMC4458218
- DOI: 10.1016/j.cell.2015.05.009
A TRP Channel Senses Lysosome Neutralization by Pathogens to Trigger Their Expulsion
Abstract
Vertebrate cells have evolved elaborate cell-autonomous defense programs to monitor subcellular compartments for infection and to evoke counter-responses. These programs are activated by pathogen-associated pattern molecules and by various strategies intracellular pathogens employ to alter cellular microenvironments. Here, we show that, when uropathogenic E. coli (UPEC) infect bladder epithelial cells (BECs), they are targeted by autophagy but avoid degradation because of their capacity to neutralize lysosomal pH. This change is detected by mucolipin TRP channel 3 (TRPML3), a transient receptor potential cation channel localized to lysosomes. TRPML3 activation then spontaneously initiates lysosome exocytosis, resulting in expulsion of exosome-encased bacteria. These studies reveal a cellular default system for lysosome homeostasis that has been co-opted by the autonomous defense program to clear recalcitrant pathogens.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
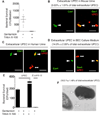
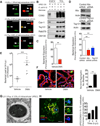

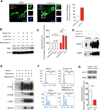
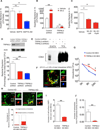

Comment in
-
Kicking Out Pathogens in Exosomes.Cell. 2015 Jun 4;161(6):1241-2. doi: 10.1016/j.cell.2015.05.040. Cell. 2015. PMID: 26046431
-
Infection: Uropathogenic Escherichia coli get a return TRP.Nat Rev Urol. 2015 Jul;12(7):362. doi: 10.1038/nrurol.2015.148. Epub 2015 Jun 16. Nat Rev Urol. 2015. PMID: 26077998 No abstract available.
References
-
- Abraham SN, Sun D, Dale JB, Beachey EH. Conservation of the D-mannose-adhesion protein among type 1 fimbriated members of the family Enterobacteriaceae. Nature. 1988;336:682–684. - PubMed
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F, Coomans C, Vermeiren E, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14:677–685. - PubMed
-
- Bishop BL, Duncan MJ, Song J, Li G, Zaas D, Abraham SN. Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat Med. 2007;13:625–630. - PubMed
-
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 DK050814/DK/NIDDK NIH HHS/United States
- R01AI35678/AI/NIAID NIH HHS/United States
- R01 AR060837/AR/NIAMS NIH HHS/United States
- R01 DK077159/DK/NIDDK NIH HHS/United States
- R01 AI50021/AI/NIAID NIH HHS/United States
- R01 HL112921/HL/NHLBI NIH HHS/United States
- R56 DK095198/DK/NIDDK NIH HHS/United States
- R01 DK101456/DK/NIDDK NIH HHS/United States
- R21 AI0561/AI/NIAID NIH HHS/United States
- R01 AI050021/AI/NIAID NIH HHS/United States
- R01 NS062792/NS/NINDS NIH HHS/United States
- R37 DK50814/DK/NIDDK NIH HHS/United States
- R21 AI056101/AI/NIAID NIH HHS/United States
- R01 AI096305/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

