Imaging patterns of fatty liver in pediatric patients
- PMID: 26027765
- PMCID: PMC4498433
- DOI: 10.5152/dir.2015.14505
Imaging patterns of fatty liver in pediatric patients
Abstract
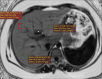
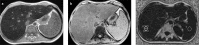
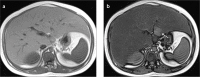
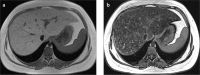
Fatty liver can present as focal, diffuse, heterogeneous, and multinodular forms. Being familiar with various patterns of steatosis can enable correct diagnosis. In patients with equivocal findings on ultrasonography, magnetic resonance imaging can be used as a problem solving tool. New techniques are promising for diagnosis and follow-up. We review imaging patterns of steatosis and new quantitative methods such as proton density fat fraction and magnetic resonance elastography for diagnosis of nonalcoholic fatty liver disease in children.
Figures

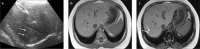
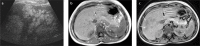
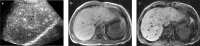
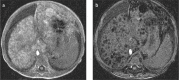
References
-
- Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. http://dx.doi.org/10.1542/peds.2006-1212. - DOI - PubMed
-
- Moran JR, Ghishan FK, Halter SA, Greene HL. Steatohepatitis in obese children: a cause of chronic liver dysfunction. Am J Gastroenterol. 1983;78:374–377. - PubMed
-
- Barshop NJ, Sırlin CB, Schwimmer JB, Lavine JE. Review article: epidemiology, pathogenesis and potential treatments of paediatric non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2008;28:13–24. http://dx.doi.org/10.1111/j.1365-2036.2008.03703.x. - DOI - PubMed
-
- Widhalm K, Ghods E. Nonalcoholic fatty liver disease: a challenge for pediatricians. Int J Obes. 2010;34:1451–1467. http://dx.doi.org/10.1038/ijo.2010.185. - DOI - PubMed
-
- Schwimmer HB, Behling C, Newbury R, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005;42:641–649. http://dx.doi.org/10.1002/hep.20842. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

