Autism-like Deficits in Shank3-Deficient Mice Are Rescued by Targeting Actin Regulators
- PMID: 26027926
- PMCID: PMC4464902
- DOI: 10.1016/j.celrep.2015.04.064
Autism-like Deficits in Shank3-Deficient Mice Are Rescued by Targeting Actin Regulators
Abstract
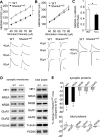
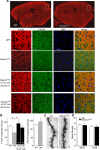
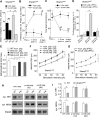
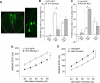
Haploinsufficiency of the Shank3 gene, which encodes a scaffolding protein at glutamatergic synapses, is a highly prevalent and penetrant risk factor for autism. Using combined behavioral, electrophysiological, biochemical, imaging, and molecular approaches, we find that Shank3-deficient mice exhibit autism-like social deficits and repetitive behaviors, as well as the significantly diminished NMDA receptor (NMDAR) synaptic function and synaptic distribution in prefrontal cortex. Concomitantly, Shank3-deficient mice have a marked loss of cortical actin filaments, which is associated with the reduced Rac1/PAK activity and increased activity of cofilin, the major actin depolymerizing factor. The social deficits and NMDAR hypofunction are rescued by inhibiting cofilin or activating Rac1 in Shank3-deficient mice and are induced by inhibiting PAK or Rac1 in wild-type mice. These results indicate that the aberrant regulation of synaptic actin filaments and loss of synaptic NMDARs contribute to the manifestation of autism-like phenotypes. Thus, targeting actin regulators provides a strategy for autism treatment.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, et al. Treatment of ischemic brain damage by perturbing NMDA receptor-PSD-95 protein interactions. Science. 2002;298:846–850. - PubMed
-
- Allen KM, Gleeson JG, Bagrodia S, Partington MW, MacMillan JC, et al. PAK3 mutation in nonsyndromic X-linked mental retardation. Nat Genet. 1998;20:25–30. - PubMed
-
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat Neurosci. 1999;2:1032–1037. - PubMed
-
- Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–809. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

