Excellent clinical outcomes and retention in care for adults with HIV-associated Kaposi sarcoma treated with systemic chemotherapy and integrated antiretroviral therapy in rural Malawi
- PMID: 26028156
- PMCID: PMC4450240
- DOI: 10.7448/IAS.18.1.19929
Excellent clinical outcomes and retention in care for adults with HIV-associated Kaposi sarcoma treated with systemic chemotherapy and integrated antiretroviral therapy in rural Malawi
Abstract
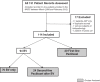
Introduction: HIV-associated Kaposi sarcoma (HIV-KS) is the most common cancer in Malawi. In 2008, the non-governmental organization, Partners In Health, and the Ministry of Health established the Neno Kaposi Sarcoma Clinic (NKSC) to treat HIV-KS in rural Neno district. We aimed to evaluate 12-month clinical outcomes and retention in care for HIV-KS patients in the NKSC, and to describe our implementation model, which featured protocol-guided chemotherapy, integrated antiretroviral therapy (ART) and psychosocial support delivered by community health workers.
Methods: We conducted a retrospective cohort study using routine clinical data from 114 adult HIV-KS patients who received ART and ≥1 chemotherapy cycle in the NKSC between March 2008 and February 2012.
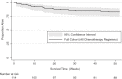
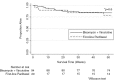
Results: At enrolment 97% of patients (n/N=103/106) had advanced HIV-KS (stage T1). Most patients were male (n/N=85/114, 75%) with median age 36 years (interquartile range, IQR: 29-42). Patients started ART a median of 77 days prior to chemotherapy (IQR: 36-252), with 97% (n/N=105/108) receiving nevirapine/lamivudine/stavudine. Following standardized protocols, we treated 20 patients (18%) with first-line paclitaxel and 94 patients (82%) with bleomycin plus vincristine (BV). Of the 94 BV patients, 24 (26%) failed to respond to BV requiring change to second-line paclitaxel. A Division of AIDS grade 3/4 adverse event occurred in 29% of patients (n/N=30/102). Neutropenia was the most common grade 3/4 event (n/N=17/102, 17%). Twelve months after chemotherapy initiation, 83% of patients (95% CI: 74-89%) were alive, including 88 (77%) retained in care. Overall survival (OS) at 12 months did not differ by initial chemotherapy regimen (p=0.6). Among patients with T1 disease, low body mass index (BMI) (adjusted hazard ratio, aHR=4.10, 95% CI: 1.06-15.89) and 1 g/dL decrease in baseline haemoglobin (aHR=1.52, 95% CI: 1.03-2.25) were associated with increased death or loss to follow-up at 12 months.
Conclusions: The NKSC model resulted in infrequent adverse events, low loss to follow-up and excellent OS. Our results suggest it is safe, effective and feasible to provide standard-of-care chemotherapy regimens from the developed world, integrated with ART, to treat HIV-KS in rural Malawi. Baseline BMI and haemoglobin may represent important patient characteristics associated with HIV-KS survival in rural sub-Saharan Africa.
Keywords: Kaposi sarcoma; Malawi; antiretroviral therapy; bleomycin; community health worker; paclitaxel; psychosocial support; vincristine.
Figures

References
-
- IARC. Sub-Saharan Africa population fact sheet. Lyon, France: International Agency for Research on Cancer; 2012.
-
- UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2013. Geneva: UNAIDS; 2013.
-
- UNAIDS. The gap report. Geneva: UNAIDS; 2014.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

