PD-1 Blockade in Tumors with Mismatch-Repair Deficiency
- PMID: 26028255
- PMCID: PMC4481136
- DOI: 10.1056/NEJMoa1500596
PD-1 Blockade in Tumors with Mismatch-Repair Deficiency
Abstract
Background: Somatic mutations have the potential to encode "non-self" immunogenic antigens. We hypothesized that tumors with a large number of somatic mutations due to mismatch-repair defects may be susceptible to immune checkpoint blockade.
Methods: We conducted a phase 2 study to evaluate the clinical activity of pembrolizumab, an anti-programmed death 1 immune checkpoint inhibitor, in 41 patients with progressive metastatic carcinoma with or without mismatch-repair deficiency. Pembrolizumab was administered intravenously at a dose of 10 mg per kilogram of body weight every 14 days in patients with mismatch repair-deficient colorectal cancers, patients with mismatch repair-proficient colorectal cancers, and patients with mismatch repair-deficient cancers that were not colorectal. The coprimary end points were the immune-related objective response rate and the 20-week immune-related progression-free survival rate.
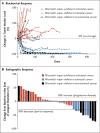
Results: The immune-related objective response rate and immune-related progression-free survival rate were 40% (4 of 10 patients) and 78% (7 of 9 patients), respectively, for mismatch repair-deficient colorectal cancers and 0% (0 of 18 patients) and 11% (2 of 18 patients) for mismatch repair-proficient colorectal cancers. The median progression-free survival and overall survival were not reached in the cohort with mismatch repair-deficient colorectal cancer but were 2.2 and 5.0 months, respectively, in the cohort with mismatch repair-proficient colorectal cancer (hazard ratio for disease progression or death, 0.10 [P<0.001], and hazard ratio for death, 0.22 [P=0.05]). Patients with mismatch repair-deficient noncolorectal cancer had responses similar to those of patients with mismatch repair-deficient colorectal cancer (immune-related objective response rate, 71% [5 of 7 patients]; immune-related progression-free survival rate, 67% [4 of 6 patients]). Whole-exome sequencing revealed a mean of 1782 somatic mutations per tumor in mismatch repair-deficient tumors, as compared with 73 in mismatch repair-proficient tumors (P=0.007), and high somatic mutation loads were associated with prolonged progression-free survival (P=0.02).
Conclusions: This study showed that mismatch-repair status predicted clinical benefit of immune checkpoint blockade with pembrolizumab. (Funded by Johns Hopkins University and others; ClinicalTrials.gov number, NCT01876511.).
Figures

Comment in
-
PD-1 Blockade in Tumors with Mismatch-Repair Deficiency.N Engl J Med. 2015 Nov 12;373(20):1979. doi: 10.1056/NEJMc1510353. N Engl J Med. 2015. PMID: 26559582 No abstract available.
-
PD-1 Blockade in Tumors with Mismatch-Repair Deficiency.N Engl J Med. 2015 Nov 12;373(20):1979. doi: 10.1056/NEJMc1510353. N Engl J Med. 2015. PMID: 26559583 No abstract available.
-
Case 23-2015: A Woman with Headache, Cognitive Impairment, and Weakness.N Engl J Med. 2015 Nov 12;373(20):1983. doi: 10.1056/NEJMc1510498. N Engl J Med. 2015. PMID: 26559591 No abstract available.
References
-
- Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–22. - PubMed
-
- Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–47. - PubMed
-
- Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–51. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- CA57345/CA/NCI NIH HHS/United States
- R01 CA067941/CA/NCI NIH HHS/United States
- R37 CA043460/CA/NCI NIH HHS/United States
- U01 CA067941/CA/NCI NIH HHS/United States
- CA16058/CA/NCI NIH HHS/United States
- P30CA006973/CA/NCI NIH HHS/United States
- P50 CA062924/CA/NCI NIH HHS/United States
- P30 CA006973/CA/NCI NIH HHS/United States
- CA67941/CA/NCI NIH HHS/United States
- CA43460/CA/NCI NIH HHS/United States
- CA163672/CA/NCI NIH HHS/United States
- P50CA062924/CA/NCI NIH HHS/United States
- R37 CA057345/CA/NCI NIH HHS/United States
- P30 CA016058/CA/NCI NIH HHS/United States
- R01 CA057345/CA/NCI NIH HHS/United States
- K23 CA163672/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
