Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer
- PMID: 26028407
- PMCID: PMC4681400
- DOI: 10.1056/NEJMoa1504627
Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer
Abstract
Background: Patients with advanced squamous-cell non-small-cell lung cancer (NSCLC) who have disease progression during or after first-line chemotherapy have limited treatment options. This randomized, open-label, international, phase 3 study evaluated the efficacy and safety of nivolumab, a fully human IgG4 programmed death 1 (PD-1) immune-checkpoint-inhibitor antibody, as compared with docetaxel in this patient population.
Methods: We randomly assigned 272 patients to receive nivolumab, at a dose of 3 mg per kilogram of body weight every 2 weeks, or docetaxel, at a dose of 75 mg per square meter of body-surface area every 3 weeks. The primary end point was overall survival.
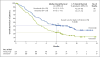
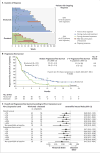
Results: The median overall survival was 9.2 months (95% confidence interval [CI], 7.3 to 13.3) with nivolumab versus 6.0 months (95% CI, 5.1 to 7.3) with docetaxel. The risk of death was 41% lower with nivolumab than with docetaxel (hazard ratio, 0.59; 95% CI, 0.44 to 0.79; P<0.001). At 1 year, the overall survival rate was 42% (95% CI, 34 to 50) with nivolumab versus 24% (95% CI, 17 to 31) with docetaxel. The response rate was 20% with nivolumab versus 9% with docetaxel (P=0.008). The median progression-free survival was 3.5 months with nivolumab versus 2.8 months with docetaxel (hazard ratio for death or disease progression, 0.62; 95% CI, 0.47 to 0.81; P<0.001). The expression of the PD-1 ligand (PD-L1) was neither prognostic nor predictive of benefit. Treatment-related adverse events of grade 3 or 4 were reported in 7% of the patients in the nivolumab group as compared with 55% of those in the docetaxel group.
Conclusions: Among patients with advanced, previously treated squamous-cell NSCLC, overall survival, response rate, and progression-free survival were significantly better with nivolumab than with docetaxel, regardless of PD-L1 expression level. (Funded by Bristol-Myers Squibb; CheckMate 017 ClinicalTrials.gov number, NCT01642004.).
Figures
Comment in
-
Lung cancer: Squiring immunotherapy to CheckMate.Nat Rev Clin Oncol. 2015 Aug;12(8):436. doi: 10.1038/nrclinonc.2015.110. Epub 2015 Jun 30. Nat Rev Clin Oncol. 2015. PMID: 26122186 No abstract available.
References
-
- Travis WD. Pathology of lung cancer. Clin Chest Med. 2011;32:669–92. - PubMed
-
- Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens: the TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol. 2000;18:2354–62. - PubMed
-
- Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–103. - PubMed
-
- Taxotere (docetaxel) U S prescribing information. Bridgewater, NJ: Sanofi-Aventis; May 2010, http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020449s059lbl.pdf.
-
- National Comprehensive Cancer Network (NCCN) Non-small cell lung cancer guidelines, v4. 2015 http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#nscl.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
