Finding the Wolf in Sheep's Clothing: The 4Kscore Is a Novel Blood Test That Can Accurately Identify the Risk of Aggressive Prostate Cancer
- PMID: 26028995
- PMCID: PMC4444768
Finding the Wolf in Sheep's Clothing: The 4Kscore Is a Novel Blood Test That Can Accurately Identify the Risk of Aggressive Prostate Cancer
Abstract
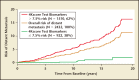
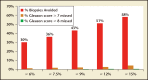
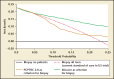
Better biomarkers that can discriminate between aggressive and indolent phenotypes of prostate cancer are urgently needed. In the first 20 years of the prostate-specific antigen (PSA) era, screening for prostate cancer has successfully reduced prostate cancer mortality, but has led to significant problems with overdiagnosis and overtreatment. As a result, many men are subjected to unnecessary prostate biopsies and overtreatment of indolent cancer in order to save one man from dying of prostate cancer. A novel blood test known as the 4Kscore® Test (OPKO Lab, Nashville, TN) incorporates a panel of four kallikrein protein biomarkers (total PSA, free PSA, intact PSA, and human kallikrein-related peptidase 2) and other clinical information in an algorithm that provides a percent risk for a high-grade (Gleason score ≥ 7) cancer on biopsy. In 10 peer-reviewed publications, the four kallikrein biomarkers and algorithm of the 4Kscore Test have been shown to improve the prediction not only of biopsy histopathology, but also surgical pathology and occurrence of aggressive, metastatic disease. Recently, a blinded prospective trial of the 4Kscore Test was conducted across the United States among 1012 men. The 4Kscore Test replicated previous European results showing accuracy in predicting biopsy outcome of Gleason score ≥ 7. In a recent case-control study nested within a population-based cohort from Västerbotten, Sweden, the four kallikrein biomarkers of the 4Kscore Test also predicted the risk for aggressive prostate cancer that metastasized within 20 years after the test was administered. These results indicate that men with an abnormal PSA or digital rectal examination result, and for whom an initial or repeat prostate biopsy is being considered, would benefit from a reflex 4Kscore Test to add important information to the clinical decision-making process. A high-risk 4Kscore Test result may be used to select men with a high probability of aggressive prostate cancer who would benefit from a biopsy of the prostate to prevent an adverse and potentially lethal outcome from prostate cancer. Men with a low 4Kscore Test result may safely defer biopsy.
Keywords: Biomarker; High-grade prostate cancer; Prostate cancer; Screening.
Figures


References
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. - PubMed
-
- Schröder FH, Hugosson J, Roobol MJ, et al. ERSPC Investigators. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. - PubMed
-
- Nam RK, Saskin R, Lee Y, et al. Increasing hospital admission rates for urological complications after transrectal ultrasound guided prostate biopsy. J Urol. 2013;189(1 suppl):S12–S18. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
