Age- and sex-dependent susceptibility to phenobarbital-resistant neonatal seizures: role of chloride co-transporters
- PMID: 26029047
- PMCID: PMC4429249
- DOI: 10.3389/fncel.2015.00173
Age- and sex-dependent susceptibility to phenobarbital-resistant neonatal seizures: role of chloride co-transporters
Abstract
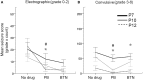
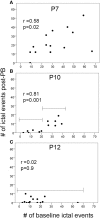
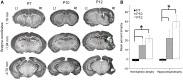
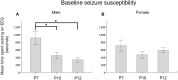
Ischemia in the immature brain is an important cause of neonatal seizures. Temporal evolution of acquired neonatal seizures and their response to anticonvulsants are of great interest, given the unreliability of the clinical correlates and poor efficacy of first-line anti-seizure drugs. The expression and function of the electroneutral chloride co-transporters KCC2 and NKCC1 influence the anti-seizure efficacy of GABAA-agonists. To investigate ischemia-induced seizure susceptibility and efficacy of the GABAA-agonist phenobarbital (PB), with NKCC1 antagonist bumetanide (BTN) as an adjunct treatment, we utilized permanent unilateral carotid-ligation to produce acute ischemic-seizures in post-natal day 7, 10, and 12 CD1 mice. Immediate post-ligation video-electroencephalograms (EEGs) quantitatively evaluated baseline and post-treatment seizure burdens. Brains were examined for stroke-injury and western blot analyses to evaluate the expression of KCC2 and NKCC1. Severity of acute ischemic seizures post-ligation was highest at P7. PB was an efficacious anti-seizure agent at P10 and P12, but not at P7. BTN failed as an adjunct, at all ages tested and significantly blunted PB-efficacy at P10. Significant acute post-ischemic downregulation of KCC2 was detected at all ages. At P7, males displayed higher age-dependent seizure susceptibility, associated with a significant developmental lag in their KCC2 expression. This study established a novel neonatal mouse model of PB-resistant seizures that demonstrates age/sex-dependent susceptibility. The age-dependent profile of KCC2 expression and its post-insult downregulation may underlie the PB-resistance reported in this model. Blocking NKCC1 with low-dose BTN following PB treatment failed to improve PB-efficacy.
Keywords: KCC2; NKCC1; bumetanide; ischemia; neonatal seizures; phenobarbital.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

