Nuclear entry of DNA viruses
- PMID: 26029198
- PMCID: PMC4429625
- DOI: 10.3389/fmicb.2015.00467
Nuclear entry of DNA viruses
Abstract
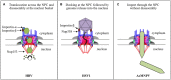
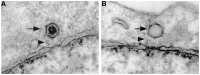
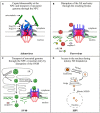
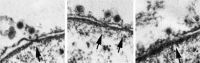
DNA viruses undertake their replication within the cell nucleus, and therefore they must first deliver their genome into the nucleus of their host cells. Thus, trafficking across the nuclear envelope is at the basis of DNA virus infections. Nuclear transport of molecules with diameters up to 39 nm is a tightly regulated process that occurs through the nuclear pore complex (NPC). Due to the enormous diversity of virus size and structure, each virus has developed its own strategy for entering the nucleus of their host cells, with no two strategies alike. For example, baculoviruses target their DNA-containing capsid to the NPC and subsequently enter the nucleus intact, while the hepatitis B virus capsid crosses the NPC but disassembles at the nuclear side of the NPC. For other viruses such as herpes simplex virus and adenovirus, although both dock at the NPC, they have each developed a distinct mechanism for the subsequent delivery of their genome into the nucleus. Remarkably, other DNA viruses, such as parvoviruses and human papillomaviruses, access the nucleus through an NPC-independent mechanism. This review discusses our current understanding of the mechanisms used by DNA viruses to deliver their genome into the nucleus, and further presents the experimental evidence for such mechanisms.
Keywords: DNA virus; Nuclear import; nuclear envelope; nuclear pore complex; nucleoporins; virus nuclear entry; virus nuclear import.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

