Structural properties of scaffolds: Crucial parameters towards stem cells differentiation
- PMID: 26029344
- PMCID: PMC4444613
- DOI: 10.4252/wjsc.v7.i4.728
Structural properties of scaffolds: Crucial parameters towards stem cells differentiation
Abstract
Tissue engineering is a multidisciplinary field that applies the principles of engineering and life-sciences for regeneration of damaged tissues. Stem cells have attracted much interest in tissue engineering as a cell source due to their ability to proliferate in an undifferentiated state for prolonged time and capability of differentiating to different cell types after induction. Scaffolds play an important role in tissue engineering as a substrate that can mimic the native extracellular matrix and the properties of scaffolds have been shown to affect the cell behavior such as the cell attachment, proliferation and differentiation. Here, we focus on the recent reports that investigated the various aspects of scaffolds including the materials used for scaffold fabrication, surface modification of scaffolds, topography and mechanical properties of scaffolds towards stem cells differentiation effect. We will present a more detailed overview on the effect of mechanical properties of scaffolds on stem cells fate.
Keywords: Differentiation; Mechanical properties; Stem cells; Surface modification; Tissue engineering; Topography.
Figures
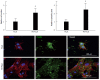
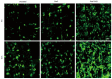
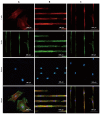
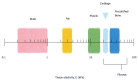

References
-
- Prabhakaran MP, Venugopal J, Ghasemi-Mobarakeh L, Kai D, Jin G, Ramakrishna S. Stem cells and nanostructures for advanced tissue regeneration. Biomedical applications of polymeric nanofibers. Berlin: Springer; 2012. pp. 21–62.
-
- Li Y, Rodrigues J, Tomás H. Injectable and biodegradable hydrogels: gelation, biodegradation and biomedical applications. Chem Soc Rev. 2012;41:2193–2221. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

