Posttranslational modifications of α-tubulin in alzheimer disease
- PMID: 26029362
- PMCID: PMC4448339
- DOI: 10.1186/s40035-015-0030-4
Posttranslational modifications of α-tubulin in alzheimer disease
Abstract
Background: In Alzheimer disease (AD), hyperphosphorylation of tau proteins results in microtubule destabilization and cytoskeletal abnormalities. Our prior ultra-morphometric studies documented a clear reduction in microtubules in pyramidal neurons in AD compared to controls, however, this reduction did not coincide with the presence of paired helical filaments. The latter suggests the presence of compensatory mechanism(s) that stabilize microtubule dynamics despite the loss of tau binding and stabilization. Microtubules are composed of tubulin dimers which are subject to posttranslational modifications that affect the stability and function of microtubules.
Methods: In this study, we performed a detailed analysis on changes in the posttranslational modifications in tubulin in postmortem human brain tissues from AD patients and age-matched controls by immunoblot and immunocytochemistry.
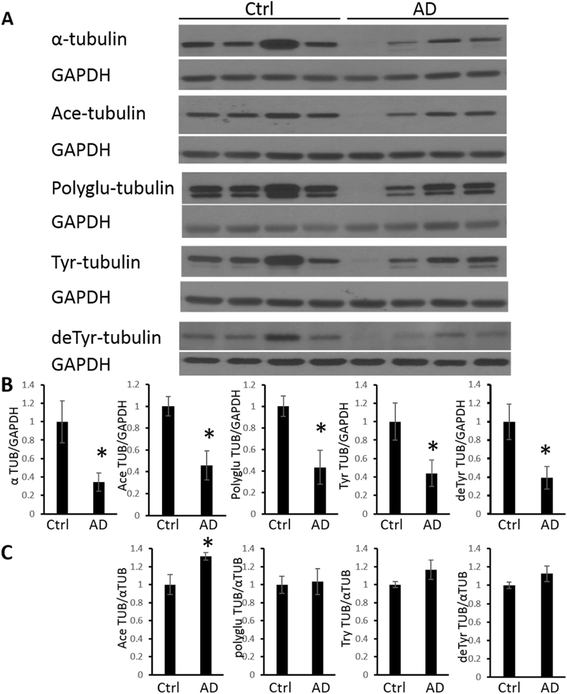
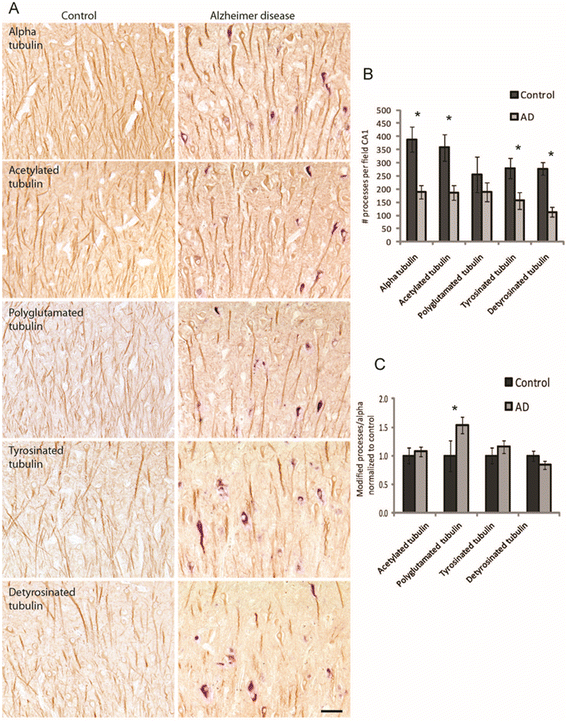
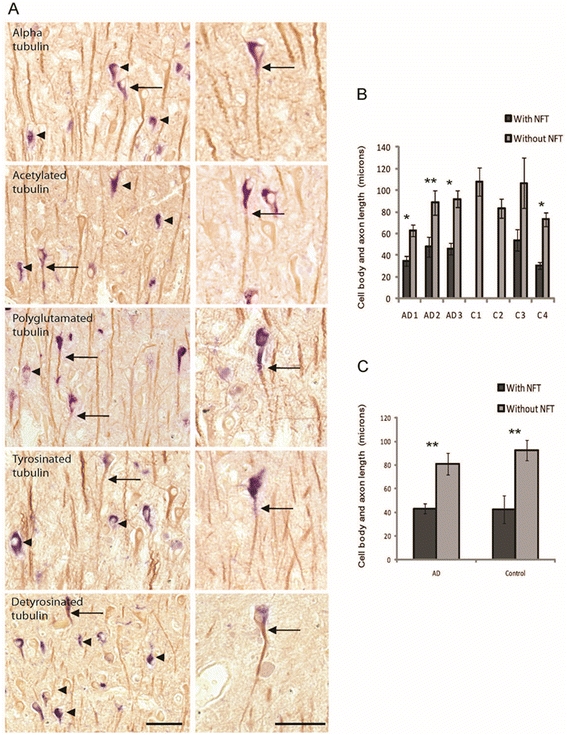
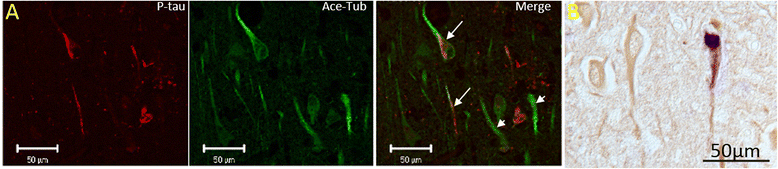
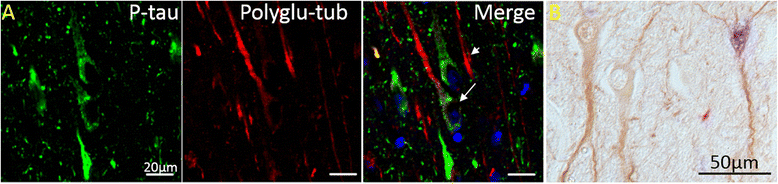
Results: Consistent with our previous study, we found decreased levels of α-tubulin in AD brain. Levels of tubulin with various posttranslational modifications such as polyglutamylation, tyrosination, and detyrosination were also proportionally reduced in AD brain, but, interestingly, there was an increase in the proportion of the acetylated α-tubulin in the remaining α-tubulin. Tubulin distribution was changed from predominantly in the processes to be more accumulated in the cell body. The number of processes containing polyglutamylated tubulin was well preserved in AD neurons. While there was a cell autonomous detrimental effect of NFTs on tubulin, this is likely a gradual and slow process, and there was no selective loss of acetylated or polyglutamylated tubulin in NFT-bearing neurons.
Conclusions: Overall, we suggest that the specific changes in tubulin modification in AD brain likely represent a compensatory response.
Keywords: Acetylation; Alzheimer disease; Polyglutamylation; Tau; Tubulin.
Figures
References
-
- Wang JZ, Xia YY, Grundke-Iqbal I, Iqbal K. Abnormal hyperphosphorylation of tau: sites, regulation, and molecular mechanism of neurofibrillary degeneration. J Alzheimers Dis. 2013;33(Suppl 1):S123–39. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

