Comparative impact of AAV and enzyme replacement therapy on respiratory and cardiac function in adult Pompe mice
- PMID: 26029718
- PMCID: PMC4445006
- DOI: 10.1038/mtm.2015.7
Comparative impact of AAV and enzyme replacement therapy on respiratory and cardiac function in adult Pompe mice
Abstract
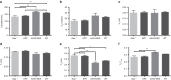
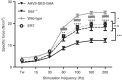
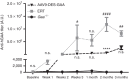
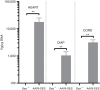
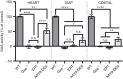
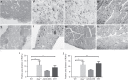
Pompe disease is an autosomal recessive genetic disorder characterized by a deficiency of the enzyme responsible for degradation of lysosomal glycogen (acid α-glucosidase (GAA)). Cardiac dysfunction and respiratory muscle weakness are primary features of this disorder. To attenuate the progressive and rapid accumulation of glycogen resulting in cardiorespiratory dysfunction, adult Gaa (-/-) mice were administered a single systemic injection of rAAV2/9-DES-hGAA (AAV9-DES) or bimonthly injections of recombinant human GAA (enzyme replacement therapy (ERT)). Assessment of cardiac function and morphology was measured 1 and 3 months after initiation of treatment while whole-body plethysmography and diaphragmatic contractile function was evaluated at 3 months post-treatment in all groups. Gaa (-/-) animals receiving either AAV9-DES or ERT demonstrated a significant improvement in cardiac function and diaphragmatic contractile function as compared to control animals. AAV9-DES treatment resulted in a significant reduction in cardiac dimension (end diastolic left ventricular mass/gram wet weight; EDMc) at 3 months postinjection. Neither AAV nor ERT therapy altered minute ventilation during quiet breathing (eupnea). However, breathing frequency and expiratory time were significantly improved in AAV9-DES animals. These results indicate systemic delivery of either strategy improves cardiac function but AAV9-DES alone improves respiratory parameters at 3 months post-treatment in a murine model of Pompe disease.
Figures

References
-
- Fukuda T, Ewan L, Bauer M, Mattaliano RJ, Zaal K, Ralston E. Dysfunction of endocytic and autophagic pathways in a lysosomal storage disease. Ann Neurol. 2006;59:700–708. - PubMed
-
- Raben N, Danon M, Gilbert AL, Dwivedi S, Collins B, Thurberg BL. Enzyme replacement therapy in the mouse model of Pompe disease. Mol Genet Metab. 2003;80:159–169. - PubMed
-
- Raben N, Nagaraju K, Lee E, Kessler P, Byrne B, Lee L. Targeted disruption of the acid alpha-glucosidase gene in mice causes an illness with critical features of both infantile and adult human glycogen storage disease type II. J Biol Chem. 1998;273:19086–19092. - PubMed
-
- Raben N, Takikita S, Pittis MG, Bembi B, Marie SK, Roberts A. Deconstructing Pompe disease by analyzing single muscle fibers: to see a world in a grain of sand. Autophagy. 2007;3:546–552. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

