Reversal of diabetes following transplantation of an insulin-secreting human liver cell line: Melligen cells
- PMID: 26029722
- PMCID: PMC4445011
- DOI: 10.1038/mtm.2015.11
Reversal of diabetes following transplantation of an insulin-secreting human liver cell line: Melligen cells
Abstract
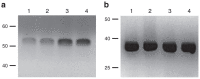
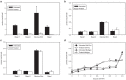
As an alternative to the transplantation of islets, a human liver cell line has been genetically engineered to reverse type 1 diabetes (TID). The initial liver cell line (Huh7ins) commenced secretion of insulin in response to a glucose concentration of 2.5 mmol/l. After transfection of the Huh7ins cells with human islet glucokinase, the resultant Melligen cells secreted insulin in response to glucose within the physiological range; commencing at 4.25 mmol/l. Melligen cells exhibited increased glucokinase enzymatic activity in response to physiological glucose concentrations, as compared with Huh7ins cells. When transplanted into diabetic immunoincompetent mice, Melligen cells restored normoglycemia. Quantitative real-time polymerase chain reaction (qRT-PCR) revealed that both cell lines expressed a range of β-cell transcription factors and pancreatic hormones. Exposure of Melligen and Huh7ins cells to proinflammatory cytokines (TNF-α, IL-1β, and IFN-γ) affected neither their viability nor their ability to secrete insulin to glucose. Gene expression (microarray and qRT-PCR) analyses indicated the survival of Melligen cells in the presence of known β-cell cytotoxins was associated with the expression of NF-κB and antiapoptotic genes (such as BIRC3). This study describes the successful generation of an artificial β-cell line, which, if encapsulated to avoid allograft rejection, may offer a clinically applicable cure for T1D.
Figures





References
-
- Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med. 1986;314:1360–1368. - PubMed
-
- Paty BW, Ryan EA, Shapiro AM, Lakey JR, Robertson RP. Intrahepatic islet transplantation in type 1 diabetic patients does not restore hypoglycemic hormonal counterregulation or symptom recognition after insulin independence. Diabetes. 2002;51:3428–3434. - PubMed
-
- Simpson AM, Marshall GM, Tuch BE, Maxwell L, Szymanska B, Tu J. Gene therapy of diabetes: glucose-stimulated insulin secretion in a human hepatoma cell line (HEP G2ins/g) Gene Ther. 1997;4:1202–1215. - PubMed
-
- Tuch BE, Szymanska B, Yao M, Tabiin MT, Gross DJ, Holman S. Function of a genetically modified human liver cell line that stores, processes and secretes insulin. Gene Ther. 2003;10:490–503. - PubMed
-
- Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med. 2000;6:568–572. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

