γ-Secretase Inhibitors and Modulators Induce Distinct Conformational Changes in the Active Sites of γ-Secretase and Signal Peptide Peptidase
- PMID: 26030233
- PMCID: PMC5436900
- DOI: 10.1021/acschembio.5b00321
γ-Secretase Inhibitors and Modulators Induce Distinct Conformational Changes in the Active Sites of γ-Secretase and Signal Peptide Peptidase
Abstract
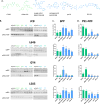
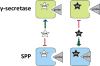
γ-Secretase inhibitors (GSIs) and modulators (GSMs) are at the frontline of cancer and Alzheimer's disease research, respectively. While both are therapeutically promising, not much is known about their interactions with proteins other than γ-secretase. Signal peptide peptidase (SPP), like γ-secretase, is a multispan transmembrane aspartyl protease that catalyzes regulated intramembrane proteolysis. We used active site-directed photophore walking probes to study the effects of different GSIs and GSMs on the active sites of γ-secretase and SPP and found that nontransition state GSIs inhibit labeling of γ-secretase by activity-based probes but enhance labeling of SPP. The opposite is true of GSMs, which have little effect on the labeling of γ-secretase but diminish labeling of SPP. These results demonstrate that GSIs and GSMs are altering the structure of not only γ-secretase but also SPP, leading to potential changes in enzyme activity and specificity that may impact the clinical outcomes of these molecules.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
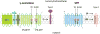


References
-
- Brown MS, Ye J, Rawson RB, Goldstein JL. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell. 2000;100:391–398. - PubMed
-
- Lemberg MK, Bland FA, Weihofen A, Braud VM, Martoglio B. Intramembrane proteolysis of signal peptides: an essential step in the generation of HLA-E epitopes. J Immunol. 2001;167:6441–6446. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

