Combinatorial Immunoprofiling in Latent Tuberculosis Infection. Toward Better Risk Stratification
- PMID: 26030344
- PMCID: PMC4595688
- DOI: 10.1164/rccm.201412-2141OC
Combinatorial Immunoprofiling in Latent Tuberculosis Infection. Toward Better Risk Stratification
Abstract
Rationale: Most immunocompetent patients diagnosed with latent tuberculosis infection (LTBI) will not progress to tuberculosis (TB) reactivation. However, current diagnostic tools cannot reliably distinguish nonprogressing from progressing patients a priori, and thus LTBI therapy must be prescribed with suboptimal patient specificity. We hypothesized that LTBI diagnostics could be improved by generating immunomarker profiles capable of categorizing distinct patient subsets by a combinatorial immunoassay approach.
Objectives: A combinatorial immunoassay analysis was applied to identify potential immunomarker combinations that distinguish among unexposed subjects, untreated patients with LTBI, and treated patients with LTBI and to differentiate risk of reactivation.
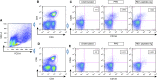
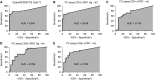
Methods: IFN-γ release assay (IGRA) was combined with a flow cytometric assay that detects induction of CD25(+)CD134(+) coexpression on TB antigen-stimulated T cells from peripheral blood. The combinatorial immunoassay analysis was based on receiver operating characteristic curves, technical cut-offs, 95% bivariate normal density ellipse prediction, and statistical analysis. Risk of reactivation was estimated with a prediction formula.
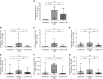
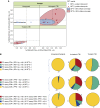
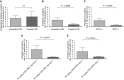
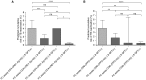
Measurements and main results: Sixty-five out of 150 subjects were included. The combinatorial immunoassay approach identified at least four different T-cell subsets. The representation of these immune phenotypes was more heterogeneous in untreated patients with LTBI than in treated patients with LTBI or unexposed groups. Patients with IGRA(+) CD4(+)CD25(+)CD134(+) T-cell phenotypes had the highest estimated reactivation risk (4.11 ± 2.11%).
Conclusions: These findings suggest that immune phenotypes defined by combinatorial assays may potentially have a role in identifying those at risk of developing TB; this potential role is supported by risk of reactivation modeling. Prospective studies will be needed to test this novel approach.
Keywords: biomarker; flow cytometry; immunoassay; latent tuberculosis infection; tuberculosis.
Figures
Comment in
-
First Steps in Biomarker Assessment for Reactivation Risk in Latent Tuberculosis Infection.Am J Respir Crit Care Med. 2015 Sep 1;192(5):534-5. doi: 10.1164/rccm.201506-1119ED. Am J Respir Crit Care Med. 2015. PMID: 26325153 No abstract available.
References
-
- Comstock GW, Baum C, Snider DE., Jr Isoniazid prophylaxis among Alaskan Eskimos: a final report of the bethel isoniazid studies. Am Rev Respir Dis. 1979;119:827–830. - PubMed
-
- Canetti G. Dynamic aspects of the pathology and bacteriology of tuberculous lesions. Am Rev Tuberc. 1956;74:13–21; discussion, 22–27. - PubMed
-
- Mack U, Migliori GB, Sester M, Rieder HL, Ehlers S, Goletti D, Bossink A, Magdorf K, Hölscher C, Kampmann B, et al. C. Lange; TBNET. LTBI: latent tuberculosis infection or lasting immune responses to M. tuberculosis? A TBNET consensus statement. Eur Respir J. 2009;33:956–973. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

