Unraveling the molecular architecture of a G protein-coupled receptor/β-arrestin/Erk module complex
- PMID: 26030356
- PMCID: PMC4649906
- DOI: 10.1038/srep10760
Unraveling the molecular architecture of a G protein-coupled receptor/β-arrestin/Erk module complex
Abstract
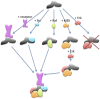
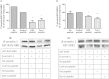
β-arrestins serve as signaling scaffolds downstream of G protein-coupled receptors, and thus play a crucial role in a plethora of cellular processes. Although it is largely accepted that the ability of β-arrestins to interact simultaneously with many protein partners is key in G protein-independent signaling of GPCRs, only the precise knowledge of these multimeric arrangements will allow a full understanding of the dynamics of these interactions and their functional consequences. However, current experimental procedures for the determination of the three-dimensional structures of protein-protein complexes are not well adapted to analyze these short-lived, multi-component assemblies. We propose a model of the receptor/β-arrestin/Erk1 signaling module, which is consistent with most of the available experimental data. Moreover, for the β-arrestin/Raf1 and the β-arrestin/ERK interactions, we have used the model to design interfering peptides and shown that they compete with both partners, hereby demonstrating the validity of the predicted interaction regions.
Figures
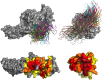


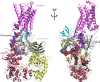
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

