Circulating Hemocytes from Larvae of the Japanese Rhinoceros Beetle Allomyrina dichotoma (Linnaeus) (Coleoptera: Scarabaeidae) and the Cellular Immune Response to Microorganisms
- PMID: 26030396
- PMCID: PMC4452365
- DOI: 10.1371/journal.pone.0128519
Circulating Hemocytes from Larvae of the Japanese Rhinoceros Beetle Allomyrina dichotoma (Linnaeus) (Coleoptera: Scarabaeidae) and the Cellular Immune Response to Microorganisms
Abstract
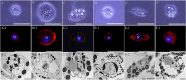
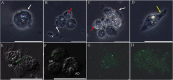
Hemocytes of the last larva of the Japanese rhinoceros beetle A. dichotoma (Linnaeus) (Coleoptera: Scarabaeidae) were classified as granulocytes, plasmatocytes, oenocytoids, spherulocytes, prohemocytes, and adipohemocytes. Among these cell types, only the granulocytes became immunologically activated with obvious morphological changes, displaying large amoeba-like, lobopodia-like, and fan-like structures. In addition, their cytoplasmic granules became larger and greatly increased in number. To explore whether these granules could be immunologically generated as phagosomes, total hemocytes were stained with LysoTracker. Greater than 90% of the granulocytes retained the LysoTracker dye at 4 h post-bacterial infection. In flow cytometry analysis, the red fluorescent signal was highly increased at 4 h post-bacterial infection (60.36%) compared to controls (5.08%), as was confirmed by fluorescent microscopy. After 12 h post-infection, these signals returned to basal levels. The uptake of pathogens by granulocytes rapidly triggered the translocation of the microtubule-associated protein 1 light chain 3 alpha (LC3) to the phagosome, which may result in enhanced pathogen killing.
Conflict of interest statement
Figures



References
-
- Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster . Annu Rev Immunol. 2007; 25: 697–743. - PubMed
-
- Strand MR. The insect cellular immune response. Insect science. 2008; 15: 1−14.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

