Group B streptococcal infection and activation of human astrocytes
- PMID: 26030618
- PMCID: PMC4452173
- DOI: 10.1371/journal.pone.0128431
Group B streptococcal infection and activation of human astrocytes
Abstract
Background: Streptococcus agalactiae (Group B Streptococcus, GBS) is the leading cause of life-threatening meningitis in human newborns in industrialized countries. Meningitis results from neonatal infection that occurs when GBS leaves the bloodstream (bacteremia), crosses the blood-brain barrier (BBB), and enters the central nervous system (CNS), where the bacteria contact the meninges. Although GBS is known to invade the BBB, subsequent interaction with astrocytes that physically associate with brain endothelium has not been well studied.
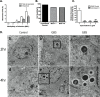
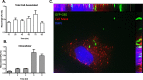
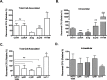
Methodology/principal findings: We hypothesize that human astrocytes play a unique role in GBS infection and contribute to the development of meningitis. To address this, we used a well- characterized human fetal astrocyte cell line, SVG-A, and examined GBS infection in vitro. We observed that all GBS strains of representative clinically dominant serotypes (Ia, Ib, III, and V) were able to adhere to and invade astrocytes. Cellular invasion was dependent on host actin cytoskeleton rearrangements, and was specific to GBS as Streptococcus gordonii failed to enter astrocytes. Analysis of isogenic mutant GBS strains deficient in various cell surface organelles showed that anchored LTA, serine-rich repeat protein (Srr1) and fibronectin binding (SfbA) proteins all contribute to host cell internalization. Wild-type GBS also displayed an ability to persist and survive within an intracellular compartment for at least 12 h following invasion. Moreover, GBS infection resulted in increased astrocyte transcription of interleukin (IL)-1β, IL-6 and VEGF.
Conclusions/significance: This study has further characterized the interaction of GBS with human astrocytes, and has identified the importance of specific virulence factors in these interactions. Understanding the role of astrocytes during GBS infection will provide important information regarding BBB disruption and the development of neonatal meningitis.
Conflict of interest statement
Figures
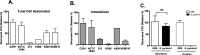

References
-
- Chin K, Fitzhhardinge P. Sequelae of early-onset group B hemolytic streptococcal neonatal meningitis. J Pediatr. 1985;106(5):819–22. - PubMed
-
- Edwards MS RM, Haffar AA, Murphy MA, Desmond MM, Baker CJ. Long-term sequelae of group B streptococcal meningitis in infants. J Pediatr 1985;106(5):717–22. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

