β-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation
- PMID: 26030850
- PMCID: PMC4482795
- DOI: 10.1038/nn.4035
β-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation
Abstract
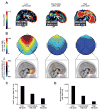
Independent evidence associates β-amyloid pathology with both non-rapid eye movement (NREM) sleep disruption and memory impairment in older adults. However, whether the influence of β-amyloid pathology on hippocampus-dependent memory is, in part, driven by impairments of NREM slow wave activity (SWA) and associated overnight memory consolidation is unknown. Here we show that β-amyloid burden in medial prefrontal cortex (mPFC) correlates significantly with the severity of impairment in NREM SWA generation. Moreover, reduced NREM SWA generation was further associated with impaired overnight memory consolidation and impoverished hippocampal-neocortical memory transformation. Furthermore, structural equation models revealed that the association between mPFC β-amyloid pathology and impaired hippocampus-dependent memory consolidation was not direct, but instead statistically depended on the intermediary factor of diminished NREM SWA. By linking β-amyloid pathology with impaired NREM SWA, these data implicate sleep disruption as a mechanistic pathway through which β-amyloid pathology may contribute to hippocampus-dependent cognitive decline in the elderly.
Figures


Comment in
-
Sleep: Amyloid-β accumulation impairs memory by disrupting deep sleep--but could the vicious cycle be stopped?Nat Rev Neurol. 2015 Jul;11(7):368. doi: 10.1038/nrneurol.2015.102. Epub 2015 Jun 9. Nat Rev Neurol. 2015. PMID: 26055468 No abstract available.
-
How amyloid, sleep and memory connect.Nat Neurosci. 2015 Jul;18(7):933-4. doi: 10.1038/nn.4048. Nat Neurosci. 2015. PMID: 26108720 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

